Inter-action between maleic acid and N- R-furfuryl-amines: crystal structure of 2-methyl- N-[(5-phenyl-furan-2-yl)meth-yl]propan-2-aminium (2 Z)-3-carb-oxy-acrylate and N-[(5-iodo-furan-2-yl)meth-yl]-2-methyl-propan-2-aminium (2 Z)-3-carb-oxy-prop-2-enoate
- PMID: 28435710
- PMCID: PMC5382611
- DOI: 10.1107/S2056989017003541
Inter-action between maleic acid and N- R-furfuryl-amines: crystal structure of 2-methyl- N-[(5-phenyl-furan-2-yl)meth-yl]propan-2-aminium (2 Z)-3-carb-oxy-acrylate and N-[(5-iodo-furan-2-yl)meth-yl]-2-methyl-propan-2-aminium (2 Z)-3-carb-oxy-prop-2-enoate
Abstract
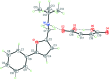
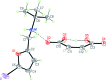
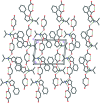
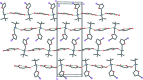
The title mol-ecular salts, C15H20NO+·C4H3O4-, (I), and C9H15INO+·C4H3O4-, (II), have very similar mol-ecular geometries for both cation and anion. The anions of both (I) and (II) are practically planar (r.m.s. deviations = 0.062 and 0.072 Å, respectively) and adopt a rare symmetrical geometry with the hy-droxy H atom approximately equidistant from the two O atoms. In their crystals, the cations and anions in both (I) and (II) form tight ionic pairs via strong N-H⋯O hydrogen bonds, with a roughly perpendicular disposition of the anion to the furan ring of the cation. This ion-pair conformation appears to correlate with the lack of reactivity of these salts in [4 + 2] cyclo-addition reactions. In the extended structures of (I) and (II), the ion pairs form hydrogen-bonded chains propagating along [010] and [001], respectively, via N-H⋯O hydrogen bonds.
Keywords: Diels–Alder reaction; crystal structure; furans; maleates; synchrotron radiation.
Figures
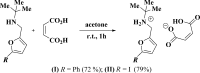
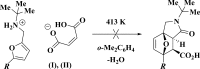
References
-
- Berson, J. A. & Swidler, R. (1953). J. Am. Chem. Soc. 75, 1721–1726.
-
- Berson, J. A. & Swidler, R. (1954). J. Am. Chem. Soc. 76, 4060–4069.
-
- Brown, T. H. (1986). Patent US4567176A1.
-
- Bruker (2001). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Other Literature Sources
