A Clinical Trial Simulation Evaluating Epinephrine Pharmacokinetics at various Dosing Frequencies during Cardiopulmonary Resuscitation
- PMID: 28435854
- PMCID: PMC5399886
A Clinical Trial Simulation Evaluating Epinephrine Pharmacokinetics at various Dosing Frequencies during Cardiopulmonary Resuscitation
Abstract
Objective: This article seeks to test the hypothesis that repeated 1mg intravenous epinephrine dosing intervals of 3-minutes and 5-minutes results in differences in the total drug exposure and the maximum epinephrine concentration using simulated cardiopulmonary resuscitation (CPR) dosing.
Methods: Published population pharmacokinetic parameters were identified in the literature and pharmacokinetic dosing simulations were conducted according to the 2015 American Heart Association guidelines for CPR in adults. The stochastic pharmacokinetic simulations were conducted in MATLAB and R for statistical programming.
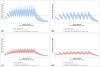
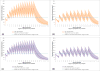
Results: A total of 5000 simulations were conducted in MATLAB while 90,000 data points for the 3-minute dosing interval and 150,000 data points for the 5-minute epinephrine dosing interval resulted from pharmacokinetic simulations in R. The difference between the 3-minute and 5-minute dosing intervals for patients with a SAP score of 30, were found to be: Male ΔAUC=2416 and ΔCmax=71, Female ΔAUC=1422 and ΔCmax=41, and for a 70kg patient ΔAUC=2968 and ΔCmax=90. While in virtual healthy participants, the differences were calculated to be ΔAUC=2658 and ΔCmax=81 for 3-minute and 5-minute dosing frequencies.
Conclusions: Epinephrine plasma levels during a simulated CPR scenario in a virtual patient population are dependent upon intravenous dosing intervals of either 3-minutes or 5-minutes. Based on the results of this clinical trial simulation, implications may exist that may require clinical studies investigating the influence of the 1mg epinephrine dosing frequency on the return of spontaneous circulation.
Keywords: CPR; clinical trial simulation; epinephrine; pharmacokinetics.
Conflict of interest statement
Conflicts of Interest: The author declares no conflict of interest.
Figures


Similar articles
-
Epinephrine dosing strategies during pediatric extracorporeal cardiopulmonary resuscitation reveal novel impacts on survival: A multicenter study utilizing time-stamped epinephrine dosing records.Resuscitation. 2023 Jul;188:109855. doi: 10.1016/j.resuscitation.2023.109855. Epub 2023 May 29. Resuscitation. 2023. PMID: 37257678 Free PMC article.
-
2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support.Pediatrics. 2006 May;117(5):e989-1004. doi: 10.1542/peds.2006-0219. Pediatrics. 2006. PMID: 16651298
-
Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial.JAMA. 2013 Jul 17;310(3):270-9. doi: 10.1001/jama.2013.7832. JAMA. 2013. PMID: 23860985 Clinical Trial.
-
[The use of arginine vasopressin during cardiopulmonary resuscitation. An analysis of experimental and clinical experience and a view of the future].Anaesthesist. 2002 Mar;51(3):191-202. doi: 10.1007/s00101-002-0287-8. Anaesthesist. 2002. PMID: 11993081 Review. German.
-
What is the right dose of epinephrine?Pediatr Crit Care Med. 2005 Sep;6(5):592-4. doi: 10.1097/01.pcc.0000170608.04086.49. Pediatr Crit Care Med. 2005. PMID: 16148823 Review.
Cited by
-
Advanced pharmacodynamics of cangrelor in healthy volunteers: a dose-finding, open-label, pilot trial.Thromb J. 2022 Apr 14;20(1):19. doi: 10.1186/s12959-022-00377-z. Thromb J. 2022. PMID: 35422039 Free PMC article.
References
-
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol Rev. 2004;56 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
