Microbial arms race: Ballistic "nematocysts" in dinoflagellates represent a new extreme in organelle complexity
- PMID: 28435864
- PMCID: PMC5375639
- DOI: 10.1126/sciadv.1602552
Microbial arms race: Ballistic "nematocysts" in dinoflagellates represent a new extreme in organelle complexity
Abstract
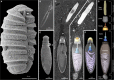
We examine the origin of harpoon-like secretory organelles (nematocysts) in dinoflagellate protists. These ballistic organelles have been hypothesized to be homologous to similarly complex structures in animals (cnidarians); but we show, using structural, functional, and phylogenomic data, that nematocysts evolved independently in both lineages. We also recorded the first high-resolution videos of nematocyst discharge in dinoflagellates. Unexpectedly, our data suggest that different types of dinoflagellate nematocysts use two fundamentally different types of ballistic mechanisms: one type relies on a single pressurized capsule for propulsion, whereas the other type launches 11 to 15 projectiles from an arrangement similar to a Gatling gun. Despite their radical structural differences, these nematocysts share a single origin within dinoflagellates and both potentially use a contraction-based mechanism to generate ballistic force. The diversity of traits in dinoflagellate nematocysts demonstrates a stepwise route by which simple secretory structures diversified to yield elaborate subcellular weaponry.
Keywords: Convergent evolution; cnidocyst; extrusome; minicollagen; mucocyst; red queen; secretion; secretory; trichocyst.
Figures



References
-
- Hausmann K., Extrusive organelles in protists. Int. Rev. Cytol. 52, 197–276 (1978). - PubMed
-
- Schmoker C., Hernández-León S., Calbet A., Microzooplankton grazing in the oceans: Impacts, data variability, knowledge gaps and future directions. J. Plankton Res. 35, 691–706 (2013).
-
- Matsuoka K., Cho H.-J., Jacobson D. M., Observations of the feeding behavior and growth rates of the heterotrophic dinoflagellate Polykrikos kofoidii (Polykrikaceae, Dinophyceae). Phys. Chem. Chem. Phys. 39, 82–86 (2000).
-
- Lee M. J., Jeong H. J., Lee K. H., Jang S. H., Kim J. H., Kim K. Y., Mixotrophy in the nematocyst–taeniocyst complex-bearing phototrophic dinoflagellate Polykrikos hartmannii. Harmful Algae 49, 124–134 (2015).
-
- Westfall J. A., Bradbury P. C., The fine structure of the nematocyst taeniocyst complex in polykrikos-kofoidi. J. Protozool. 29, 474–475 (1982).
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

