Layered microporous polymers by solvent knitting method
- PMID: 28435866
- PMCID: PMC5376128
- DOI: 10.1126/sciadv.1602610
Layered microporous polymers by solvent knitting method
Abstract
Two-dimensional (2D) nanomaterials, especially 2D organic nanomaterials with unprecedentedly diverse and controlled structure, have attracted decent scientific interest. Among the preparation strategies, the top-down approach is one of the considered low-cost and scalable strategies to obtain 2D organic nanomaterials. However, some factors of their layered counterparts limited the development and potential applications of 2D organic nanomaterials, such as type, stability, and strict synthetic conditions of layered counterparts. We report a class of layered solvent knitting hyper-cross-linked microporous polymers (SHCPs) prepared by improving Friedel-Crafts reaction and using dichloroalkane as an economical solvent, stable electrophilic reagent, and external cross-linker at low temperature, which could be used as layered counterparts to obtain previously unknown 2D SHCP nanosheets by method of ultrasonic-assisted solvent exfoliation. This efficient and low-cost strategy can produce previously unreported microporous organic polymers with layered structure and high surface area and gas storage capacity. The pore structure and surface area of these polymers can be controlled by tuning the chain length of the solvent, the molar ratio of AlCl3, and the size of monomers. Furthermore, we successfully obtain an unprecedentedly high-surface area HCP material (3002 m2 g-1), which shows decent gas storage capacity (4.82 mmol g-1 at 273 K and 1.00 bar for CO2; 12.40 mmol g-1 at 77.3 K and 1.13 bar for H2). This finding provides an opportunity for breaking the constraint of former knitting methods and opening up avenues for the design and synthesis of previously unknown layered HCP materials.
Keywords: Friedel-Crafts reaction; Gas storage; High surface area; Hypercrosslinked polymers; Knitting method; Microporous polymer; Naonosheets; Ordered stucture; Two-dimensional.
Figures
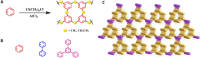





Similar articles
-
Knitting polycyclic aromatic hydrocarbon-based microporous organic polymers for efficient CO2 capture.RSC Adv. 2018 Mar 13;8(19):10347-10354. doi: 10.1039/c8ra01332b. eCollection 2018 Mar 13. RSC Adv. 2018. PMID: 35540478 Free PMC article.
-
Two-Dimensional Porous Polymers: From Sandwich-like Structure to Layered Skeleton.Acc Chem Res. 2018 Dec 18;51(12):3191-3202. doi: 10.1021/acs.accounts.8b00444. Epub 2018 Nov 9. Acc Chem Res. 2018. PMID: 30411885
-
Nanostructured Hypercrosslinked Porous Organic Polymers: Morphological Evolution and Rapid Separation of Polar Organic Micropollutants.ACS Appl Mater Interfaces. 2022 Feb 9;14(5):7369-7381. doi: 10.1021/acsami.1c24393. Epub 2022 Jan 28. ACS Appl Mater Interfaces. 2022. PMID: 35089681
-
Controlled growth of organic 2D layered material thin films via interfacial methods.Chem Commun (Camb). 2022 Nov 8;58(89):12384-12398. doi: 10.1039/d2cc03941a. Chem Commun (Camb). 2022. PMID: 36278437 Review.
-
Two-Dimensional Microporous Material-based Mixed Matrix Membranes for Gas Separation.Chem Asian J. 2020 Aug 3;15(15):2303-2315. doi: 10.1002/asia.202000053. Epub 2020 Mar 5. Chem Asian J. 2020. PMID: 32039557 Review.
Cited by
-
Single Molecular Layer of Chitin Sub-Nanometric Nanoribbons: One-Pot Self-Exfoliation and Crystalline Assembly into Robust, Sustainable, and Moldable Structural Materials.Adv Sci (Weinh). 2022 May;9(16):e2201287. doi: 10.1002/advs.202201287. Epub 2022 Mar 31. Adv Sci (Weinh). 2022. PMID: 35355436 Free PMC article.
-
Carbon dioxide adsorption based on porous materials.RSC Adv. 2021 Mar 31;11(21):12658-12681. doi: 10.1039/d0ra10902a. eCollection 2021 Mar 29. RSC Adv. 2021. PMID: 35423803 Free PMC article. Review.
-
Synthesis of Novel Phenyl Porous Organic Polymers and Their Excellent Visible Light Photocatalytic Performance on Antibiotics.Materials (Basel). 2019 Oct 11;12(20):3296. doi: 10.3390/ma12203296. Materials (Basel). 2019. PMID: 31614425 Free PMC article.
-
Advances in Conjugated Microporous Polymers.Chem Rev. 2020 Feb 26;120(4):2171-2214. doi: 10.1021/acs.chemrev.9b00399. Epub 2020 Jan 28. Chem Rev. 2020. PMID: 31990527 Free PMC article.
-
Selective photocatalytic CO2 reduction in aerobic environment by microporous Pd-porphyrin-based polymers coated hollow TiO2.Nat Commun. 2022 Mar 17;13(1):1400. doi: 10.1038/s41467-022-29102-0. Nat Commun. 2022. PMID: 35301319 Free PMC article.
References
-
- Geim A. K., Graphene: Status and prospects. Science 324, 1530–1534 (2009). - PubMed
-
- Pakdel A., Bando Y., Golberg D., Nano boron nitride flatland. Chem. Soc. Rev. 43, 934–959 (2014). - PubMed
-
- Ma R., Sasaki T., Nanosheets of oxides and hydroxides: Ultimate 2D charge-bearing functional crystallites. Adv. Mater. 22, 5082–5104 (2010). - PubMed
-
- Bilbao N., Destoop I., De Feyter S., González-Rodríguez D., Two-dimensional nanoporous networks formed by liquid-to-solid transfer of hydrogen-bonded macrocycles built from DNA bases. Angew. Chem. Int. Ed. 55, 659–663 (2016). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

