Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation
- PMID: 28435870
- PMCID: PMC5381952
- DOI: 10.1126/sciadv.1601556
Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation
Abstract
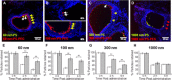
Mucoadhesive particles (MAP) have been widely explored for pulmonary drug delivery because of their perceived benefits in improving particle residence in the lungs. However, retention of particles adhesively trapped in airway mucus may be limited by physiologic mucus clearance mechanisms. In contrast, particles that avoid mucoadhesion and have diameters smaller than mucus mesh spacings rapidly penetrate mucus layers [mucus-penetrating particles (MPP)], which we hypothesized would provide prolonged lung retention compared to MAP. We compared in vivo behaviors of variously sized, polystyrene-based MAP and MPP in the lungs following inhalation. MAP, regardless of particle size, were aggregated and poorly distributed throughout the airways, leading to rapid clearance from the lungs. Conversely, MPP as large as 300 nm exhibited uniform distribution and markedly enhanced retention compared to size-matched MAP. On the basis of these findings, we formulated biodegradable MPP (b-MPP) with an average diameter of <300 nm and examined their behavior following inhalation relative to similarly sized biodegradable MAP (b-MAP). Although b-MPP diffused rapidly through human airway mucus ex vivo, b-MAP did not. Rapid b-MPP movements in mucus ex vivo correlated to a more uniform distribution within the airways and enhanced lung retention time as compared to b-MAP. Furthermore, inhalation of b-MPP loaded with dexamethasone sodium phosphate (DP) significantly reduced inflammation in a mouse model of acute lung inflammation compared to both carrier-free DP and DP-loaded MAP. These studies provide a careful head-to-head comparison of MAP versus MPP following inhalation and challenge a long-standing dogma that favored the use of MAP for pulmonary drug delivery.
Keywords: biodegradable nanoparticles; controlled release; inhaled drug delivery; lung inflammation; mucociliary clearance; mucus-penetrating particles.
Figures




Similar articles
-
Enhanced pulmonary delivery of fluticasone propionate in rodents by mucus-penetrating nanoparticles.Int J Pharm. 2016 Apr 11;502(1-2):188-97. doi: 10.1016/j.ijpharm.2016.02.031. Epub 2016 Feb 20. Int J Pharm. 2016. PMID: 26902722
-
Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo.ACS Nano. 2015 Sep 22;9(9):9217-27. doi: 10.1021/acsnano.5b03876. Epub 2015 Aug 31. ACS Nano. 2015. PMID: 26301576 Free PMC article.
-
Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse.J Control Release. 2015 Jan 10;197:48-57. doi: 10.1016/j.jconrel.2014.10.026. Epub 2014 Nov 4. J Control Release. 2015. PMID: 25449804 Free PMC article.
-
Airway mucus in pulmonary diseases: Muco-adhesive and muco-penetrating particles to overcome the airway mucus barriers.Int J Pharm. 2023 Mar 5;634:122661. doi: 10.1016/j.ijpharm.2023.122661. Epub 2023 Feb 1. Int J Pharm. 2023. PMID: 36736964 Free PMC article. Review.
-
Technological strategies to estimate and control diffusive passage times through the mucus barrier in mucosal drug delivery.Adv Drug Deliv Rev. 2018 Jan 15;124:64-81. doi: 10.1016/j.addr.2017.12.002. Epub 2017 Dec 12. Adv Drug Deliv Rev. 2018. PMID: 29246855 Free PMC article. Review.
Cited by
-
pH-Responsive Polymer Nanoparticles for Efficient Delivery of Cas9 Ribonucleoprotein With or Without Donor DNA.Adv Mater. 2022 Jun;34(23):e2110618. doi: 10.1002/adma.202110618. Epub 2022 Apr 28. Adv Mater. 2022. PMID: 35119139 Free PMC article.
-
Targeted delivery of antibiotics to the infected pulmonary tissues using ROS-responsive nanoparticles.J Nanobiotechnology. 2019 Oct 3;17(1):103. doi: 10.1186/s12951-019-0537-4. J Nanobiotechnology. 2019. PMID: 31581948 Free PMC article.
-
Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting.Int J Mol Sci. 2023 Feb 8;24(4):3390. doi: 10.3390/ijms24043390. Int J Mol Sci. 2023. PMID: 36834804 Free PMC article. Review.
-
Codelivery of synergistic antimicrobials with polyelectrolyte nanocomplexes to treat bacterial biofilms and lung infections.Sci Adv. 2023 Jan 20;9(3):eade8039. doi: 10.1126/sciadv.ade8039. Epub 2023 Jan 20. Sci Adv. 2023. PMID: 36662850 Free PMC article.
-
Targeted Nanocarrier Delivery of RNA Therapeutics to Control HIV Infection.Pharmaceutics. 2022 Jun 26;14(7):1352. doi: 10.3390/pharmaceutics14071352. Pharmaceutics. 2022. PMID: 35890248 Free PMC article. Review.
References
-
- Azarmi S., Roa W. H., Löbenberg R., Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 60, 863–875 (2008). - PubMed
-
- Yang W., Peters J. I., Williams R. O. III, Inhaled nanoparticles—A current review. Int. J. Pharm. 356, 239–247 (2008). - PubMed
-
- Edwards D. A., Ben-Jebria A., Langer R., Recent advances in pulmonary drug delivery using large, porous inhaled particles. J. Appl. Physiol. 85, 379–385 (1998). - PubMed
-
- Panyam J., Labhasetwar V., Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55, 329–347 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

