Elucidating the Aβ42 Anti-Aggregation Mechanism of Action of Tramiprosate in Alzheimer's Disease: Integrating Molecular Analytical Methods, Pharmacokinetic and Clinical Data
- PMID: 28435985
- PMCID: PMC5488121
- DOI: 10.1007/s40263-017-0434-z
Elucidating the Aβ42 Anti-Aggregation Mechanism of Action of Tramiprosate in Alzheimer's Disease: Integrating Molecular Analytical Methods, Pharmacokinetic and Clinical Data
Abstract
Background: Amyloid beta (Aβ) oligomers play a critical role in the pathogenesis of Alzheimer's disease (AD) and represent a promising target for drug development. Tramiprosate is a small-molecule Aβ anti-aggregation agent that was evaluated in phase III clinical trials for AD but did not meet the primary efficacy endpoints; however, a pre-specified subgroup analysis revealed robust, sustained, and clinically meaningful cognitive and functional effects in patients with AD homozygous for the ε4 allele of apolipoprotein E4 (APOE4/4 homozygotes), who carry an increased risk for the disease. Therefore, to build on this important efficacy attribute and to further improve its pharmaceutical properties, we have developed a prodrug of tramiprosate ALZ-801 that is in advanced stages of clinical development. To elucidate how tramiprosate works, we investigated its molecular mechanism of action (MOA) and the translation to observed clinical outcomes.
Objective: The two main objectives of this research were to (1) elucidate and characterize the MOA of tramiprosate via an integrated application of three independent molecular methodologies and (2) present an integrated translational analysis that links the MOA, conformation of the target, stoichiometry, and pharmacokinetic dose exposure to the observed clinical outcome in APOE4/4 homozygote subjects.
Method: We used three molecular analytical methods-ion mobility spectrometry-mass spectrometry (IMS-MS), nuclear magnetic resonance (NMR), and molecular dynamics-to characterize the concentration-related interactions of tramiprosate versus Aβ42 monomers and the resultant conformational alterations affecting aggregation into oligomers. The molecular stoichiometry of the tramiprosate versus Aβ42 interaction was further analyzed in the context of clinical pharmacokinetic dose exposure and central nervous system Aβ42 levels (i.e., pharmacokinetic-pharmacodynamic translation in humans).
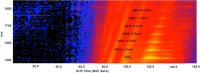
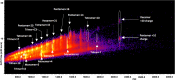
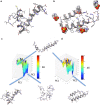
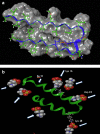
Results: We observed a multi-ligand interaction of tramiprosate with monomeric Aβ42, which differs from the traditional 1:1 binding. This resulted in the stabilization of Aβ42 monomers and inhibition of oligomer formation and elongation, as demonstrated by IMS-MS and molecular dynamics. Using NMR spectroscopy and molecular dynamics, we also showed that tramiprosate bound to Lys16, Lys28, and Asp23, the key amino acid side chains of Aβ42 that are responsible for both conformational seed formation and neuronal toxicity. The projected molar excess of tramiprosate versus Aβ42 in humans using the dose effective in patients with AD aligned with the molecular stoichiometry of the interaction, providing a clear clinical translation of the MOA. A consistent alignment of these preclinical-to-clinical elements describes a unique example of translational medicine and supports the efficacy seen in symptomatic patients with AD. This unique "enveloping mechanism" of tramiprosate also provides a potential basis for tramiprosate dose selection for patients with homozygous AD at earlier stages of disease.
Conclusion: We have identified the molecular mechanism that may account for the observed clinical efficacy of tramiprosate in patients with APOE4/4 homozygous AD. In addition, the integrated application of the molecular methodologies (i.e., IMS-MS, NMR, and thermodynamics analysis) indicates that it is feasible to modulate and control the Aβ42 conformational dynamics landscape by a small molecule, resulting in a favorable Aβ42 conformational change that leads to a clinically relevant amyloid anti-aggregation effect and inhibition of oligomer formation. This novel enveloping MOA of tramiprosate has potential utility in the development of disease-modifying therapies for AD and other neurodegenerative diseases caused by misfolded proteins.
Conflict of interest statement
Funding
Alzheon Inc. sponsored the research described in this article and paid the open access fee.
Conflict of interest
PK, MT, JH, and JY are employees of Alzheon Inc. WS and SR are employees of Schrödinger, which had a scientific services agreement in place to perform some of the work in this manuscript. KB has served as a consultant or on advisory boards for Alzheon, Eli Lilly, Fujirebio Europe, IBL International, Novartis, and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. HF has no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

