Antibiotic therapy for pelvic inflammatory disease
- PMID: 28436019
- PMCID: PMC6478260
- DOI: 10.1002/14651858.CD010285.pub2
Antibiotic therapy for pelvic inflammatory disease
Update in
-
Antibiotic therapy for pelvic inflammatory disease.Cochrane Database Syst Rev. 2020 Aug 20;8(8):CD010285. doi: 10.1002/14651858.CD010285.pub3. Cochrane Database Syst Rev. 2020. PMID: 32820536 Free PMC article.
Abstract
Background: Pelvic inflammatory disease (PID) is an infection that affects 4% to 12% of young women, and is one of the most common causes of morbidity in this age group. The main intervention for acute PID is the use of broad-spectrum antibiotics which cover Chlamydia trachomatis, Neisseria gonorrhoeae, and anaerobic bacteria, administered intravenously, intramuscularly, or orally. In this review, we assessed the optimal treatment regimen for PID.
Objectives: To assess the effectiveness and safety of antibiotic regimens used to treat pelvic inflammatory disease.
Search methods: We searched the Cochrane Sexually Transmitted Infections Review Group's Specialized Register, which included randomized controlled trials (RCTs) from 1944 to 2016, located through electronic searching and handsearching; the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid platform (1991 to July 2016); MEDLINE (1946 to July 2016); Embase (1947 to July 2016); LILACS, iAHx interface (1982 to July 2016); World Health Organization International Clinical Trials Registry Platform (July 2016); Web of Science (2001 to July 2016); OpenGrey (1990, 1992, 1995, 1996, and 1997); and abstracts in selected publications.
Selection criteria: We included RCTs comparing the use of antibiotics with placebo or other antibiotics for the treatment of PID in women of reproductive age, either as inpatient or outpatient treatment. We limited our review to comparison of drugs in current use that are recommended for consideration by the 2015 US Centers for Disease Control and Prevention (CDC) guidelines for treatment of PID.
Data collection and analysis: At least two review authors independently selected trials for inclusion, extracted data, and assessed risk of bias. We contacted investigators to obtain missing information. We resolved disagreements by consensus or by consulting a fourth review author if necessary. We assessed the quality of the evidence using GRADE criteria, classifying it as high, moderate, low, or very low. We calculated Mantel-Haenszel risk ratios (RR), using either random-effects or fixed-effect models and number needed to treat for an additional beneficial outcome or for an additional harmful outcome, with their 95% confidence interval (CI), to measure the effect of the treatments. We conducted sensitivity analyses limited to studies at low risk of bias, for comparisons where such studies were available.
Main results: We included 37 RCTs (6348 women). The quality of the evidence ranged from very low to high, the main limitations being serious risk of bias (due to poor reporting of study methods and lack of blinding), serious inconsistency, and serious imprecision. Azithromycin versus doxycyclineThere was no clear evidence of a difference between the two drugs in rates of cure for mild-moderate PID (RR 1.18, 95% CI 0.89 to 1.55, I2 = 72%, 2 RCTs, 243 women, very low-quality evidence), severe PID (RR 1.00, 95% CI 0.96 to 1.05, 1 RCT, 309 women, low-quality evidence), or adverse effects leading to discontinuation of treatment (RR 0.71, 95% CI 0.38 to 1.34, 3 RCTs, 552 women, I2 = 0%, low-quality evidence). In a sensitivity analysis limited to a single study at low risk of bias, azithromycin was superior to doxycycline in achieving cure in mild-moderate PID (RR 1.35, 95% CI 1.10 to 1.67, 133 women, moderate-quality evidence). Quinolone versus cephalosporinThere was no clear evidence of a difference between the two drugs in rates of cure for mild-moderate PID (RR 1.04, 95% CI 0.98 to 1.10, 3 RCTs, 459 women, I2 = 5%, low-quality evidence), severe PID (RR 1.06, 95% CI 0.91 to 1.23, 2 RCTs, 313 women, I2 = 7%, low-quality evidence), or adverse effects leading to discontinuation of treatment (RR 2.24, 95% CI 0.52 to 9.72, 5 RCTs, 772 women, I2 = 0%, very low-quality evidence). Nitroimidazole versus no use of nitroimidazoleThere was no conclusive evidence of a difference between the nitroimidazoles (metronidazole) group and the group receiving other drugs with activity over anaerobes (e.g. amoxicillin-clavulanate) in rates of cure for mild-moderate PID (RR 1.01, 95% CI 0.93 to 1.10, 5 RCTs, 2427 women, I2 = 60%, moderate-quality evidence), severe PID (RR 0.96, 95% CI 0.92 to 1.01, 11 RCTs, 1383 women, I2 = 0%, moderate-quality evidence), or adverse effects leading to discontinuation of treatment (RR 1.00, 95% CI 0.63 to 1.59; participants = 3788; studies = 16; I2 = 0% , low-quality evidence). In a sensitivity analysis limited to studies at low risk of bias, findings did not differ substantially from the main analysis (RR 1.06, 95% CI 0.98 to 1.15, 2 RCTs, 1201 women, I2 = 32%, high-quality evidence). Clindamycin plus aminoglycoside versus quinoloneThere was no evidence of a difference between the two groups in rates of cure for mild-moderate PID (RR 0.88, 95% CI 0.69 to 1.13, 1 RCT, 25 women, very low-quality evidence), severe PID (RR 1.02, 95% CI 0.87 to 1.19, 2 studies, 151 women, I2 = 0%, low-quality evidence), or adverse effects leading to discontinuation of treatment (RR 0.21, 95% CI 0.02 to 1.72, 3 RCTs, 163 women, very low-quality evidence). Clindamycin plus aminoglycoside versus cephalosporinThere was no clear evidence of a difference between the two groups in rates of cure for mild-moderate PID (RR 1.02, 95% CI 0.95 to 1.09, 2 RCTs, 150 women, I2 = 0%, low-quality evidence), severe PID (RR 1.00, 95% CI 0.95 to 1.06, 10 RCTs, 959 women, I2 = 21%, moderate-quality evidence), or adverse effects leading to discontinuation of treatment (RR 0.78, 95% CI 0.18 to 3.42, 10 RCTs, 1172 women, I2 = 0%, very low-quality evidence).
Authors' conclusions: We found no conclusive evidence that one regimen of antibiotics was safer or more effective than any other for the cure of PID, and there was no clear evidence for the use of nitroimidazoles (metronidazole) compared to use of other drugs with activity over anaerobes. Moderate-quality evidence from a single study at low risk of bias suggested that a macrolide (azithromycin) may be more effective than a tetracycline (doxycycline) for curing mild-moderate PID. Our review considered only the drugs that are currently used and mentioned by the CDC.
Conflict of interest statement
All authors certify that they do not have any affiliations with, or involvement in, any organization or entity with a direct financial interest in the subject matter of this review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony). They disclose that two of the authors (RFS and JR) had two publications used in the analysis. RFS and JR did not participate in the process for considering these studies for inclusion, data extraction, and grading for risk of bias.
Figures

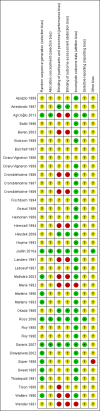
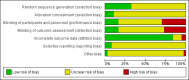








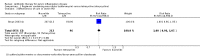













Comment in
-
What Antibiotic Regimen Is Most Efficacious in Treating Pelvic Inflammatory Disease?Ann Emerg Med. 2017 Dec;70(6):840-842. doi: 10.1016/j.annemergmed.2017.07.002. Epub 2017 Aug 17. Ann Emerg Med. 2017. PMID: 28822590 No abstract available.
References
References to studies included in this review
-
- Apuzzio JJ, Stankiewicz R, Ganesh V, Jain S, Kaminski Z, Louria D. Comparison of parenteral ciprofloxacin with clindamycin‐gentamicin in the treatment of pelvic infection. American Journal of Medicine 1989;87(5A):148S‐51S. - PubMed
-
- Arredondo JL, Diaz V, Gaitan H, Maradiegue E, Oyarzún E, Paz R, et al. Oral clindamycin and ciprofloxacin versus intramuscular ceftriaxone and oral doxycycline in the treatment of mild‐to‐moderate pelvic inflammatory disease in outpatients. Clinical Infectious Diseases 1997;24(2):170‐8. - PubMed
-
- Aşicioğlu O, Gungorduk K, Ozdemir A, Ertas IE, Yildirim G, Sanci M, et al. Single daily dose of moxifloxacin versus ofloxacin plus metronidazole as a new treatment approach to uncomplicated pelvic inflammatory disease: a multicentre prospective randomized trial. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013;171(1):116‐21. - PubMed
-
- Balbi G, Piscitelli V, Grazia F, Martini S, Balbi C, Cardone A. Acute pelvic inflammatory disease: comparison of therapeutic protocols [Malattia infiammatoria pelvica acuta: protocolli terapeutici a confronto]. Minerva Ginecologica 1996;48(1‐2):19‐23. - PubMed
-
- Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. Journal of International Medical Research 2003;31(1):45‐54. - PubMed
References to studies excluded from this review
-
- Acar B, Zissis NP. Piperacillin alone vs triple antibiotic combination in gynecological infections. Journal of chemotherapy 1989;1(6):403‐6. - PubMed
-
- Andersson PO, Hackl H, Jensen P, Larsen KR. A comparison of two different dosages of pivampicillin and doxycycline in patients with gynaecological infections. Current Medical Research and Opinion 1980;6(8):513‐7. - PubMed
-
- Bartlett JG. Therapeutic trial of moxalactam in intra‐abdominal sepsis and gynaecological infections. Drugs under Experimental and Clinical Research 1982;VIII(5):483‐6.
-
- Berkeley AS, Freedman KS, Hirsch JC, Ledger WJ. Randomized, comparative trial of imipenem/cilastatin and moxalactam in the treatment of serious obstetric and gynecologic infections. Surgery, Gynecology & Obstetrics 1986;162(3):204‐8. - PubMed
References to ongoing studies
-
- The Importance of Anti‐anaerobic Therapy for Acute Pelvic Inflammatory Disease (PID).. Ongoing study November 2010..
Additional references
-
- Aghaizu A, Adams EJ, Turner K, Kerry S, Hay P, Simms I, et al. What is the cost of pelvic inflammatory disease and how much could be prevented by screening for chlamydia trachomatis? Cost analysis of the Prevention of Pelvic Infection (POPI) trial. Sexually Transmitted Infections 2011;87(4):312‐7. - PubMed
-
- Baveja G, Saini S, Sangwan K, Arora DR. A study of bacterial pathogens in acute pelvic inflammatory disease. Journal of Communicable Diseases 2001;88:121‐5. - PubMed
-
- Datta SD, Torrone E, Kruszon‐Moran D, Berman S, Johnson R, Satterwhite CL, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999‐2008. Sexually Transmitted Diseases 2012;39(2):92‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

