Direct current stimulation boosts synaptic gain and cooperativity in vitro
- PMID: 28436038
- PMCID: PMC5451737
- DOI: 10.1113/JP273005
Direct current stimulation boosts synaptic gain and cooperativity in vitro
Abstract
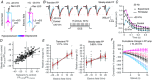
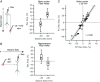
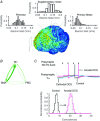
Key points: Direct current stimulation (DCS) polarity specifically modulates synaptic efficacy during a continuous train of presynaptic inputs, despite synaptic depression. DCS polarizes afferent axons and postsynaptic neurons, boosting cooperativity between synaptic inputs. Polarization of afferent neurons in upstream brain regions may modulate activity in the target brain region during transcranial DCS (tDCS). A statistical theory of coincident activity predicts that the diffuse and weak profile of current flow can be advantageous in enhancing connectivity between co-active brain regions.
Abstract: Transcranial direct current stimulation (tDCS) produces sustained and diffuse current flow in the brain with effects that are state dependent and outlast stimulation. A mechanistic explanation for tDCS should capture these spatiotemporal features. It remains unclear how sustained DCS affects ongoing synaptic dynamics and how modulation of afferent inputs by diffuse stimulation changes synaptic activity at the target brain region. We tested the effect of acute DCS (10-20 V m-1 for 3-5 s) on synaptic dynamics with constant rate (5-40 Hz) and Poisson-distributed (4 Hz mean) trains of presynaptic inputs. Across tested frequencies, sustained synaptic activity was modulated by DCS with polarity-specific effects. Synaptic depression attenuates the sensitivity to DCS from 1.1% per V m-1 to 0.55%. DCS applied during synaptic activity facilitates cumulative neuromodulation, potentially reversing endogenous synaptic depression. We establish these effects are mediated by both postsynaptic membrane polarization and afferent axon fibre polarization, which boosts cooperativity between synaptic inputs. This potentially extends the locus of neuromodulation from the nominal target to afferent brain regions. Based on these results we hypothesized the polarization of afferent neurons in upstream brain regions may modulate activity in the target brain region during tDCS. A multiscale model of transcranial electrical stimulation including a finite element model of brain current flow, numerical simulations of neuronal activity, and a statistical theory of coincident activity predicts that the diffuse and weak profile of current flow can be advantageous. Thus, we propose that specifically because tDCS is diffuse, weak and sustained it can boost connectivity between co-active brain regions.
Keywords: electrical brain stimulation; synaptic depression; transcranial direct current stimulation.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures





References
-
- Abbott LF & Regehr WG (2004). Synaptic computation. Nature 431, 796–803. - PubMed
-
- Abbott LF, Varela JA, Sen K & Nelson SB (1997). Synaptic depression and cortical gain control. Science 275, 220–224. - PubMed
-
- Antal A, Varga ET, Kincses TZ, Nitsche MA & Paulus W (2004). Oscillatory brain activity and transcranial direct current stimulation in humans. Neuroreport 15, 1307–1310. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

