Sarcomere Dysfunction in Nemaline Myopathy
- PMID: 28436394
- PMCID: PMC5467716
- DOI: 10.3233/JND-160200
Sarcomere Dysfunction in Nemaline Myopathy
Abstract
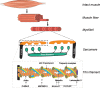
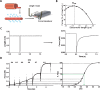
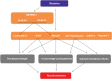
Nemaline myopathy (NM) is among the most common non-dystrophic congenital myopathies (incidence 1:50.000). Hallmark features of NM are skeletal muscle weakness and the presence of nemaline bodies in the muscle fiber. The clinical phenotype of NM patients is quite diverse, ranging from neonatal death to normal lifespan with almost normal motor function. As the respiratory muscles are involved as well, severely affected patients are ventilator-dependent. The mechanisms underlying muscle weakness in NM are currently poorly understood. Therefore, no therapeutic treatment is available yet.Eleven implicated genes have been identified: ten genes encode proteins that are either components of thin filament, or are thought to contribute to stability or turnover of thin filament proteins. The thin filament is a major constituent of the sarcomere, the smallest contractile unit in muscle. It is at this level of contraction - thin-thick filament interaction - where muscle weakness originates in NM patients.This review focusses on how sarcomeric gene mutations directly compromise sarcomere function in NM. Insight into the contribution of sarcomeric dysfunction to muscle weakness in NM, across the genes involved, will direct towards the development of targeted therapeutic strategies.
Keywords: Nemaline myopathy; contractility; thin filament.
Figures
Similar articles
-
Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency.Hum Mol Genet. 2009 Jul 1;18(13):2359-69. doi: 10.1093/hmg/ddp168. Epub 2009 Apr 4. Hum Mol Genet. 2009. PMID: 19346529 Free PMC article.
-
Deleting exon 55 from the nebulin gene induces severe muscle weakness in a mouse model for nemaline myopathy.Brain. 2013 Jun;136(Pt 6):1718-31. doi: 10.1093/brain/awt113. Epub 2013 May 28. Brain. 2013. PMID: 23715096 Free PMC article.
-
KLHL40 deficiency destabilizes thin filament proteins and promotes nemaline myopathy.J Clin Invest. 2014 Aug;124(8):3529-39. doi: 10.1172/JCI74994. Epub 2014 Jun 24. J Clin Invest. 2014. PMID: 24960163 Free PMC article.
-
Clinical and genetic heterogeneity in nemaline myopathy--a disease of skeletal muscle thin filaments.Trends Mol Med. 2001 Aug;7(8):362-8. doi: 10.1016/s1471-4914(01)02089-5. Trends Mol Med. 2001. PMID: 11516997 Review.
-
Recent advances in nemaline myopathy.Neuromuscul Disord. 2021 Oct;31(10):955-967. doi: 10.1016/j.nmd.2021.07.012. Epub 2021 Jul 24. Neuromuscul Disord. 2021. PMID: 34561123 Review.
Cited by
-
ACTA1 H40Y mutant iPSC-derived skeletal myocytes display mitochondrial defects in an in vitro model of nemaline myopathy.Exp Cell Res. 2023 Mar 15;424(2):113507. doi: 10.1016/j.yexcr.2023.113507. Epub 2023 Feb 14. Exp Cell Res. 2023. PMID: 36796746 Free PMC article.
-
Knockin mouse model of the human CFL2 p.A35T mutation results in a unique splicing defect and severe myopathy phenotype.Hum Mol Genet. 2020 Jul 29;29(12):1996-2003. doi: 10.1093/hmg/ddaa035. Hum Mol Genet. 2020. PMID: 32160286 Free PMC article.
-
Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics.Int J Mol Sci. 2021 Aug 25;22(17):9187. doi: 10.3390/ijms22179187. Int J Mol Sci. 2021. PMID: 34502093 Free PMC article. Review.
-
Drosophila Tropomodulin is required for multiple actin-dependent processes within developing myofibers.Development. 2023 Mar 15;150(6):dev201194. doi: 10.1242/dev.201194. Epub 2023 Mar 24. Development. 2023. PMID: 36806912 Free PMC article.
-
Role of MiR-325-3p in the Regulation of CFL2 and Myogenic Differentiation of C2C12 Myoblasts.Cells. 2021 Oct 12;10(10):2725. doi: 10.3390/cells10102725. Cells. 2021. PMID: 34685705 Free PMC article.
References
-
- Wallgren-Pettersson C. Congenital nemaline myopathy: A longitudinal study, vol. 30. Academic dissertation. Helsinki: The Finnish Society of Sciences and Letters, Commentationes Physico-Mathematicae 111/Dissertationes; 1990.
-
- Sanoudou D, Beggs AH. Clinical and genetic heterogeneity in nemaline myopathy-a disease of skeletal muscle thin filaments. Trends Mol Med. 2001;7(8):362–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

