Fasciola hepatica glycoconjugates immuneregulate dendritic cells through the Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin inducing T cell anergy
- PMID: 28436457
- PMCID: PMC5402274
- DOI: 10.1038/srep46748
Fasciola hepatica glycoconjugates immuneregulate dendritic cells through the Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin inducing T cell anergy
Abstract
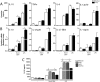
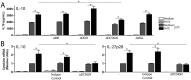
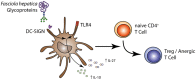
Dendritic cell-specific ICAM-3 grabbing non-integrin (DC-SIGN) expressed on a variety of DCs, is a C-type lectin receptor that recognizes glycans on a diverse range of pathogens, including parasites. The interaction of DC-SIGN with pathogens triggers specific signaling events that modulate DC-maturation and activity and regulate T-cell activation by DCs. In this work we evaluate whether F. hepatica glycans can immune modulate DCs via DC-SIGN. We demonstrate that DC-SIGN interacts with F. hepatica glycoconjugates through mannose and fucose residues. We also show that mannose is present in high-mannose structures, hybrid and trimannosyl N-glycans with terminal GlcNAc. Furthermore, we demonstrate that F. hepatica glycans induce DC-SIGN triggering leading to a strong production of TLR-induced IL-10 and IL-27p28. In addition, parasite glycans induced regulatory DCs via DC-SIGN that decrease allogeneic T cell proliferation, via the induction of anergic/regulatory T cells, highlighting the role of DC-SIGN in the regulation of innate and adaptive immune responses by F. hepatica. Our data confirm the immunomodulatory properties of DC-SIGN triggered by pathogen-derived glycans and contribute to the identification of immunomodulatory glyans of helminths that might eventually be useful for the design of vaccines against fasciolosis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Fasciola hepatica tegumental antigens induce anergic-like T cells via dendritic cells in a mannose receptor-dependent manner.Eur J Immunol. 2016 May;46(5):1180-92. doi: 10.1002/eji.201545905. Epub 2016 Mar 29. Eur J Immunol. 2016. PMID: 26931640
-
Glycans from Fasciola hepatica Modulate the Host Immune Response and TLR-Induced Maturation of Dendritic Cells.PLoS Negl Trop Dis. 2015 Dec 31;9(12):e0004234. doi: 10.1371/journal.pntd.0004234. eCollection 2015 Dec. PLoS Negl Trop Dis. 2015. PMID: 26720149 Free PMC article.
-
High molecular weight components containing N-linked oligosaccharides of Ascaris suum extract inhibit the dendritic cells activation through DC-SIGN and MR.Mol Immunol. 2017 Jul;87:33-46. doi: 10.1016/j.molimm.2017.03.015. Epub 2017 Apr 10. Mol Immunol. 2017. PMID: 28402840
-
DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe?Clin Sci (Lond). 2003 Apr;104(4):437-46. Clin Sci (Lond). 2003. PMID: 12653690 Review.
-
Pathogen recognition by DC-SIGN shapes adaptive immunity.Future Microbiol. 2009 Sep;4(7):879-90. doi: 10.2217/fmb.09.51. Future Microbiol. 2009. PMID: 19722841 Review.
Cited by
-
Fasciolosis: pathogenesis, host-parasite interactions, and implication in vaccine development.Front Vet Sci. 2023 Dec 11;10:1270064. doi: 10.3389/fvets.2023.1270064. eCollection 2023. Front Vet Sci. 2023. PMID: 38149297 Free PMC article. Review.
-
L-fucose reduces gut inflammation due to T-regulatory response in Muc2 null mice.PLoS One. 2022 Dec 30;17(12):e0278714. doi: 10.1371/journal.pone.0278714. eCollection 2022. PLoS One. 2022. PMID: 36584066 Free PMC article.
-
Helminth Antigen Exposure Enhances Early Immune Control of Mycobacterium tuberculosis in Monocytes and Macrophages.J Innate Immun. 2021;13(3):148-163. doi: 10.1159/000512279. Epub 2020 Dec 17. J Innate Immun. 2021. PMID: 33333522 Free PMC article.
-
Fasciola hepatica Fatty Acid Binding Protein 1 Modulates T cell Polarization by Promoting Dendritic Cell Thrombospondin-1 Secretion Without Affecting Metabolic Homeostasis in Obese Mice.Front Immunol. 2022 May 26;13:884663. doi: 10.3389/fimmu.2022.884663. eCollection 2022. Front Immunol. 2022. PMID: 35720355 Free PMC article.
-
DC-SIGN signalling induced by Trichinella spiralis products contributes to the tolerogenic signatures of human dendritic cells.Sci Rep. 2020 Nov 20;10(1):20283. doi: 10.1038/s41598-020-77497-x. Sci Rep. 2020. PMID: 33219293 Free PMC article.
References
-
- Garcia-Vallejo J. J. & van Kooyk Y. The physiological role of DC-SIGN: a tale of mice and men. Trends Immunol 34, 482–486 (2013). - PubMed
-
- Geijtenbeek T. B., den Dunnen J. & Gringhuis S. I. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol 4, 879–890 (2009). - PubMed
-
- Gringhuis S. I., den Dunnen J., Litjens M., van der Vlist M. & Geijtenbeek T. B. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol 10, 1081–1088 (2009). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

