Interactions of AMTN, ODAM and SCPPPQ1 proteins of a specialized basal lamina that attaches epithelial cells to tooth mineral
- PMID: 28436474
- PMCID: PMC5402393
- DOI: 10.1038/srep46683
Interactions of AMTN, ODAM and SCPPPQ1 proteins of a specialized basal lamina that attaches epithelial cells to tooth mineral
Abstract
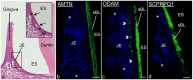
A specialized basal lamina (sBL) mediates adhesion of certain epithelial cells to the tooth. It is distinct because it does not contain collagens type IV and VII, is enriched in laminin-332, and includes three novel constituents called amelotin (AMTN), odontogenic ameloblast-associated (ODAM), and secretory calcium-binding phosphoprotein proline-glutamine rich 1 (SCPPPQ1). The objective of this study was to clarify the structural organization of the sBL. Fluorescence and immunogold labeling showed that the three proteins co-localize. Quantitative analysis of the relative position of gold particles on the sBL demonstrates that the distribution of ODAM is skewed towards the cell while that of AMTN and SCPPPQ1 tends towards the tooth surface. Bacterial two-hybrid analysis and co-immunoprecipitation, gel filtration of purified proteins and transmission electron and atomic force microscopies highlight the propensity of AMTN, ODAM, and SCPPPQ1 to interact with and among themselves and form supramolecular aggregates. These data suggest that AMTN, ODAM and SCPPPQ1 participate in structuring an extracellular matrix with the distinctive capacity of attaching epithelial cells to mineralized surfaces. This unique feature is particularly relevant for the adhesion of gingival epithelial cells to the tooth surface, which forms a protective seal that is the first line of defense against bacterial invasion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
-
- Ekblom M., Falk M., Salmivirta K., Durbeej M. & Ekblom P. Laminin isoforms and epithelial development. Annals of the New York Academy of Sciences 857, 194–211 (1998). - PubMed
-
- McGuire J. D., Walker M. P., Dusevich V., Wang Y. & Gorski J. P. Enamel organic matrix: potential structural role in enamel and relationship to residual basement membrane constituents at the dentin enamel junction. Connect. Tissue Res. 55 Suppl 1, 33–37, doi: 10.3109/03008207.2014.923883 (2014). - DOI - PMC - PubMed
-
- Takata T., Nikai H., Ijuhin N. & Okamoto H. Ultrastructure of regenerated junctional epithelium after surgery of the rat molar gingiva. J Periodontol. 57, 776–783 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

