Longitudinal magnetic resonance imaging in progressive supranuclear palsy: A new combined score for clinical trials
- PMID: 28436538
- PMCID: PMC5808453
- DOI: 10.1002/mds.26973
Longitudinal magnetic resonance imaging in progressive supranuclear palsy: A new combined score for clinical trials
Abstract
Background: Two recent, randomized, placebo-controlled phase II/III trials (clinicaltrials.gov: NCT01110720, NCT01049399) of davunetide and tideglusib in progressive supranuclear palsy (PSP) generated prospective, 1-year longitudinal datasets of high-resolution T1-weighted three-dimensional MRI.
Objective: The objective of this study was to develop a quantitative MRI disease progression measurement for clinical trials.
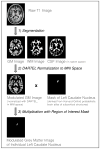
Methods: The authors performed a fully automated quantitative MRI analysis employing atlas-based volumetry and provide sample size calculations based on data collected in 99 PSP patients assigned to placebo in these trials. Based on individual volumes of 44 brain compartments and structures at baseline and 52 weeks of follow-up, means and standard deviations of annualized percentage volume changes were used to estimate standardized effect sizes and the required sample sizes per group for future 2-armed, placebo-controlled therapeutic trials.
Results: The highest standardized effect sizes were found for midbrain, frontal lobes, and the third ventricle. Using the annualized percentage volume change of these structures to detect a 50% change in the 1-year progression (80% power, significance level 5%) required lower numbers of patients per group (third ventricle, n = 32; midbrain, n = 37; frontal lobe, n = 43) than the best clinical scale (PSP rating scale total score, n = 58). A combination of volume changes in these 3 structures reduced the number of required patients to only 20 and correlated best with the progression in the clinical scales.
Conclusions: We propose the 1-year change in the volumes of third ventricle, midbrain, and frontal lobe as combined imaging read-out for clinical trials in PSP that require the least number of patients for detecting efficacy to reduce brain atrophy. © 2017 International Parkinson and Movement Disorder Society.
Keywords: clinical trials; magnetic resonance imaging; power calculation; progressive supranuclear palsy; volumetry.
© 2017 International Parkinson and Movement Disorder Society.
Figures
References
-
- Stamelou M, Höglinger G. A Review of Treatment Options for Progressive Supranuclear Palsy. CNS Drugs. 2016;30(7):629–36. - PubMed
-
- Tolosa E, Litvan I, Hoglinger GU, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014;29(4):470–478. - PubMed
-
- Höglinger GU, Huppertz HJ, Wagenpfeil S, et al. Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial. Mov Disord. 2014;29(4):479–487. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

