Pharmacologically increasing collateral perfusion during acute stroke using a carboxyhemoglobin gas transfer agent (Sanguinate™) in spontaneously hypertensive rats
- PMID: 28436705
- PMCID: PMC5987934
- DOI: 10.1177/0271678X17705567
Pharmacologically increasing collateral perfusion during acute stroke using a carboxyhemoglobin gas transfer agent (Sanguinate™) in spontaneously hypertensive rats
Abstract
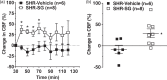
Similar to patients with chronic hypertension, spontaneously hypertensive rats (SHR) develop fast core progression during middle cerebral artery occlusion (MCAO) resulting in large final infarct volumes. We investigated the effect of Sanguinate™ (SG), a PEGylated carboxyhemoglobin (COHb) gas transfer agent, on changes in collateral and reperfusion cerebral blood flow and brain injury in SHR during 2 h of MCAO. SG (8 mL/kg) or vehicle ( n = 6-8/group) was infused i.v. after 30 or 90 min of ischemia with 2 h reperfusion. Multi-site laser Doppler probes simultaneously measured changes in core MCA and collateral flow during ischemia and reperfusion using a validated method. Brain injury was measured using TTC. Animals were anesthetized with choral hydrate. Collateral flow changed little in vehicle-treated SHR during ischemia (-8 ± 9% vs. prior to infusion) whereas flow increased in SG-treated animals (29 ± 10%; p < 0.05). In addition, SG improved reperfusion regardless of time of treatment; however, brain injury was smaller only with early treatment in SHR vs. vehicle (28.8 ± 3.2% vs. 18.8 ± 2.3%; p < 0.05). Limited collateral flow in SHR during MCAO is consistent with small penumbra and large infarction. The ability to increase collateral flow in SHR with SG suggests that this compound may be useful as an adjunct to endovascular therapy and extend the time window for treatment.
Keywords: Collateral perfusion; hypertension; infarction; ischemic stroke; reperfusion.
Figures






References
-
- Chamorro Á, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 2016; 15: 869–881. - PubMed
-
- Ginsberg MD. Expanding the concept of neuroprotection for acute ischemic stroke: The pivotal roles of reperfusion and the collateral circulation. Prog Neurobiol 2016; 145–146: 46–77. - PubMed
-
- Linfante I, Starosciak AK, Walker GR, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg 2016; 8: 224–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

