Infective respiratory syncytial virus is present in human cord blood samples and most prevalent during winter months
- PMID: 28437435
- PMCID: PMC5402929
- DOI: 10.1371/journal.pone.0173738
Infective respiratory syncytial virus is present in human cord blood samples and most prevalent during winter months
Abstract
Background: Human respiratory syncytial virus (RSV) remains the most common cause of severe lower respiratory tract disease amongst infants, and continues to cause annual epidemics of respiratory disease every winter worldwide. Demonstrating placental transmission of viable RSV in human samples is a major paradigm shift in respiratory routes considered likely for RSV transmission.
Methods: Droplet digital PCR (ddPCR) was used to identify RSV present in cord blood mononucleocytes (CBM). CBMs testing positive for RSV were treated with phytohemagglutinin (PHA), PHA and nitric oxide (NO) or PHA, NO and palivizumab, and co-cultured with HeLa cell monolayers. Subsequent immuno-staining for RSV was used to visualize infective viral plaques.
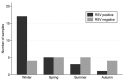
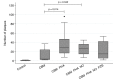
Results: RSV was detected in 26 of 45 samples (57.7%) by ddPCR. CBM's collected in winter were more likely to test positive for RSV (17/21 samples, risk = 80%, OR = 7.08; 95% CI 1.80-27.80; p = 0.005) compared to non-winter months (9/24 samples, 37.5%). RSV plaques were observed in non-treated and treated co-cultured HeLa monolayers.
Conclusions: Demonstrating active RSV in CBMs suggests in utero transmission of infective virus to the fetus without causing overt disease. This is likely to have an important impact on immune development as well as future virus-host interactions, thereby warranting further investigation.
Conflict of interest statement
Figures


References
-
- Hall CB, Simoes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Current topics in microbiology and immunology. 2013;372:39–57. doi: 10.1007/978-3-642-38919-1_2 - DOI - PubMed
-
- Everard ML. Respiratory syncytial virus bronchiolitis and pneumonia St Louis, Missouri, US: Mosby, Elsevier Health Sciences; 2008. 491–9 p.
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. PubMed Central PMCID: PMC2864404. doi: 10.1016/S0140-6736(10)60206-1 - DOI - PMC - PubMed
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. The New England journal of medicine. 2005;352(17):1749–59. doi: 10.1056/NEJMoa043951 - DOI - PubMed
-
- Hobson L, Everard ML. Persistent of respiratory syncytial virus in human dendritic cells and influence of nitric oxide. Clinical and experimental immunology. 2008;151(2):359–66. PubMed Central PMCID: PMC2276949. doi: 10.1111/j.1365-2249.2007.03560.x - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

