Relationship between early onset severe intrahepatic cholestasis of pregnancy and higher risk of meconium-stained fluid
- PMID: 28437442
- PMCID: PMC5402936
- DOI: 10.1371/journal.pone.0176504
Relationship between early onset severe intrahepatic cholestasis of pregnancy and higher risk of meconium-stained fluid
Abstract
Background: Intrahepatic cholestasis of pregnancy (ICP) is the commonest gestational liver disease. The risk of adverse fetal outcome has been associated with the severity of maternal hypercholanemia after diagnosis.
Objective: To investigate whether there is a relationship between the severity and timing of onset of hypercholanemia and the risk of meconium-stained amniotic fluid (MSAF) and adverse neonatal events.
Study design: The study included 382 pregnancies complicated by ICP managed at a referral hospital in Buenos Aires (Argentina) between June 2009 and December 2013. The patients were classified into three groups according to the severity of hypercholanemia at diagnosis; mild (10-19.9 μmol/L), moderate (20-39.9 μmol/L) and severe (≥40 μmol/L). Their clinical characteristics and pregnancy outcomes were investigated in a prospective observational study.
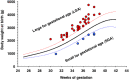
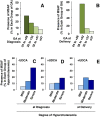
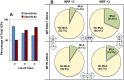
Results: Higher risk of MSAF was observed when ICP appeared early in gestation or when hypercholanemia was more severe. Taking both parameters into account an MSAF risk factor (MRF) was defined. Based on a model of positive/negative predictive values, a cut-off point of MRF = 3 was selected, which prioritized sensitivity versus specificity. In ICP patients with MRF>3, the probability of MSAF was enhanced 4-fold. An increase in the frequency of MSAF was also associated with higher serum levels at diagnosis of alanine transaminase, alkaline phosphatase and direct bilirubin.
Conclusions: The risk of MSAF is associated not only with the magnitude of hypercholanemia at diagnosis but also with the early gestational onset of raised maternal serum bile acids.
Conflict of interest statement
Figures


References
-
- Lammert F, Marschall HU, Matern S. Intrahepatic Cholestasis of Pregnancy. Curr Treat Options Gastroenterol. 2003;6: 123–32. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

