Effects of Diagnosis by Newborn Screening for Cystic Fibrosis on Weight and Length in the First Year of Life
- PMID: 28437538
- PMCID: PMC5731827
- DOI: 10.1001/jamapediatrics.2017.0206
Effects of Diagnosis by Newborn Screening for Cystic Fibrosis on Weight and Length in the First Year of Life
Abstract
Importance: Since the implementation of universal newborn screening (NBS) for cystic fibrosis (CF), the timing and magnitude of growth deficiency or its association with correlates of disease among infants with CF who underwent NBS has not been well described.
Objective: To examine incremental weight gain, linear growth, and clinical features in the first year of life among infants with CF who underwent NBS.
Design, setting, and participants: The Baby Observational and Nutrition Study (BONUS), a multicenter, longitudinal, observational cohort study, was conducted during regular CF clinic visits in the first 12 months of life at 28 US Cystic Fibrosis Foundation-accredited Care Centers from January 7, 2012, through May 31, 2015. Participants included 231 infants younger than 3.5 months who underwent NBS and had confirmed CF, with a gestational age of at least 35 weeks, birth weight of at least 2.5 kg, and toleration of full oral feeds. Of these, 222 infants (96.1%) had follow-up beyond 6 months of age and 215 (93.1%) completed 12 months of follow-up.
Exposure: Cystic fibrosis.
Main outcome and measures: Attained weight and length for age and World Health Organization normative z scores at ages 1 to 6 and 8, 10, and 12 months (defined a priori).
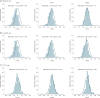
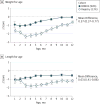
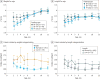
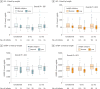
Results: Of the 231 infants enrolled, 110 infants (47.6%) were female and 121 (52.4%) were male, with a mean (SD) age of 2.58 (0.69) months. BONUS infants had lower than mean birth weights (mean z score, -0.15; 95% CI, -0.27 to -0.04) and higher birth lengths (mean z score, 0.44; 95% CI, 0.26 to 0.62). They achieved normal weight by 12 months, a significant improvement over a prescreening cohort of newborns with CF from 20 years before the contemporary cohort (mean z score increase, 0.57; 95% CI, 0.37-0.77). However, length was lower than the mean at 12 months (mean z score, -0.56; 95% CI, -0.70 to -0.42). Only 30 infants (13.6%) were at less than the 10th percentile of weight for age, whereas 53 (23.9%) were at less than the 10th percentile of length for age at more than half their visits. Male sex, pancreatic insufficiency, meconium ileus, histamine blocker use, and respiratory Pseudomonas aeruginosa infection were associated with lower weight or length during the first year. Insulinlike growth factor 1 levels were significantly lower among low-length infants. Persistently low-weight infants consumed significantly more calories, and weight and length z scores were negatively correlated with caloric intake.
Conclusions and relevance: Since initiation of universal NBS for CF, significant improvement has occurred in nutritional status, with normalization of weight in the first year of life. However, length stunting remains common.
Conflict of interest statement
Figures
References
-
- Farrell PM, Lai HJ, Li Z, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr. 2005;147(3(suppl)):S30–S36. - PubMed
-
- Farrell PM, Kosorok MR, Rock MJ, et al. Wisconsin Cystic Fibrosis Neonatal Screening Study Group Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics. 2001;107(1):1–13. - PubMed
-
- Dijk FN, McKay K, Barzi F, Gaskin KJ, Fitzgerald DA. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch Dis Child. 2011;96(12):1118–1123. - PubMed
-
- Siret D, Bretaudeau G, Branger B, et al. Comparing the clinical evolution of cystic fibrosis screened neonatally to that of cystic fibrosis diagnosed from clinical symptoms: a 10-year retrospective study in a French region (Brittany) Pediatr Pulmonol. 2003;35(5):342–349. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

