Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes
- PMID: 28437540
- PMCID: PMC5822209
- DOI: 10.1001/jamaneurol.2017.0357
Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes
Abstract
Importance: Amyotrophic lateral sclerosis (ALS) is a common adult-onset neurodegenerative disease characterized by selective loss of upper and lower motor neurons. Patients with ALS have persistent peripheral and central inflammatory responses including abnormally functioning T cells and activated microglia. However, much less is known about the inflammatory gene profile of circulating innate immune monocytes in these patients.
Objective: To characterize the transcriptomics of peripheral monocytes in patients with ALS.
Design, setting, and participants: Monocytes were isolated from peripheral blood of 43 patients with ALS and 22 healthy control individuals. Total RNA was extracted from the monocytes and subjected to deep RNA sequencing, and these results were validated by quantitative reverse transcription polymerase chain reaction.
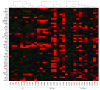
Main outcomes and measures: The differential expressed gene signatures of these monocytes were identified using unbiased RNA sequencing strategy for gene expression profiling.
Results: The demographics between the patients with ALS (mean [SD] age, 58.8 [1.57] years; 55.8% were men and 44.2% were women; 90.7% were white, 4.65% were Hispanic, 2.33% were black, and 2.33% were Asian) and control individuals were similar (mean [SD] age, 57.6 [2.15] years; 50.0% were men and 50.0% were women; 90.9% were white, none were Hispanic, none were black, and 9.09% were Asian). RNA sequencing data from negative selected monocytes revealed 233 differential expressed genes in ALS monocytes compared with healthy control monocytes. Notably, ALS monocytes demonstrated a unique inflammation-related gene expression profile, the most prominent of which, including IL1B, IL8, FOSB, CXCL1, and CXCL2, were confirmed by quantitative reverse transcription polymerase chain reaction (IL8, mean [SE], 1.00 [0.18]; P = .002; FOSB, 1.00 [0.21]; P = .009; CXCL1, 1.00 [0.14]; P = .002; and CXCL2, 1.00 [0.11]; P = .01). Amyotrophic lateral sclerosis monocytes from rapidly progressing patients had more proinflammatory DEGs than monocytes from slowly progressing patients.
Conclusions and relevance: Our data indicate that ALS monocytes are skewed toward a proinflammatory state in the peripheral circulation and may play a role in ALS disease progression, especially in rapidly progressing patients. This increased inflammatory response of peripheral immune cells may provide a potential target for disease-modifying therapy in patients with ALS.
Conflict of interest statement
Figures


Comment in
-
Motor neuron disease: Proinflammatory monocytes might contribute to ALS progression.Nat Rev Neurol. 2017 Jul;13(7):385. doi: 10.1038/nrneurol.2017.74. Epub 2017 May 12. Nat Rev Neurol. 2017. PMID: 28497803 No abstract available.
References
-
- Turner MR, Cagnin A, Turkheimer FE, et al. . Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15(3):601-609. - PubMed
-
- Henkel JS, Beers DR, Siklós L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31(3):427-437. - PubMed
-
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57(7):1282-1289. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

