Peripheral artery disease, redox signaling, oxidative stress - Basic and clinical aspects
- PMID: 28437655
- PMCID: PMC5403804
- DOI: 10.1016/j.redox.2017.04.017
Peripheral artery disease, redox signaling, oxidative stress - Basic and clinical aspects
Abstract
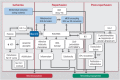
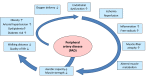
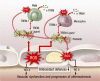
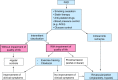
Reactive oxygen and nitrogen species (ROS and RNS, e.g. H2O2, nitric oxide) confer redox regulation of essential cellular signaling pathways such as cell differentiation, proliferation, migration and apoptosis. At higher concentrations, ROS and RNS lead to oxidative stress and oxidative damage of biomolecules (e.g. via formation of peroxynitrite, fenton chemistry). Peripheral artery disease (PAD) is characterized by severe ischemic conditions in the periphery leading to intermittent claudication and critical limb ischemia (end stage). It is well known that redox biology and oxidative stress play an important role in this setting. We here discuss the major pathways of oxidative stress and redox signaling underlying the disease progression with special emphasis on the contribution of inflammatory processes. We also highlight therapeutic strategies comprising pharmacological (e.g. statins, angiotensin-converting enzyme inhibitors, phosphodiesterase inhibition) and non-pharmacological (e.g. exercise) interventions. Both of these strategies induce potent indirect antioxidant and anti-inflammatory mechanisms that may contribute to an improvement of PAD associated complications and disease progression by removing excess formation of ROS and RNS (e.g. by ameliorating primary complications such as hyperlipidemia and hypertension) as well as the normalization of the inflammatory phenotype suppressing the progression of atherosclerosis.
Keywords: Antioxidant therapy; Claudication and critical limb ischemia; Oxidative stress; Peripheral artery (occlusive) disease; Redox signaling; Walking distance.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Interplay Between Reactive Oxygen/Reactive Nitrogen Species and Metabolism in Vascular Biology and Disease.Antioxid Redox Signal. 2021 Jun 1;34(16):1319-1354. doi: 10.1089/ars.2020.8161. Antioxid Redox Signal. 2021. PMID: 33899493 Free PMC article. Review.
-
Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations.Free Radic Biol Med. 2015 Jul;84:65-76. doi: 10.1016/j.freeradbiomed.2015.03.018. Epub 2015 Apr 2. Free Radic Biol Med. 2015. PMID: 25841784 Review.
-
Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks.Ann Bot. 2015 Sep;116(4):475-85. doi: 10.1093/aob/mcv074. Epub 2015 Jun 12. Ann Bot. 2015. PMID: 26070643 Free PMC article. Review.
-
Free radicals, metals and antioxidants in oxidative stress-induced cancer.Chem Biol Interact. 2006 Mar 10;160(1):1-40. doi: 10.1016/j.cbi.2005.12.009. Epub 2006 Jan 23. Chem Biol Interact. 2006. PMID: 16430879 Review.
-
Redox regulation of cell survival.Antioxid Redox Signal. 2008 Aug;10(8):1343-74. doi: 10.1089/ars.2007.1957. Antioxid Redox Signal. 2008. PMID: 18522489 Free PMC article. Review.
Cited by
-
Oxidative Stress and Arterial Dysfunction in Peripheral Artery Disease.Antioxidants (Basel). 2018 Oct 19;7(10):145. doi: 10.3390/antiox7100145. Antioxidants (Basel). 2018. PMID: 30347720 Free PMC article. Review.
-
Skeletal muscle desmin alterations following revascularization in peripheral artery disease claudicants.Sci Rep. 2024 Jun 1;14(1):12609. doi: 10.1038/s41598-024-63626-3. Sci Rep. 2024. PMID: 38824194 Free PMC article.
-
The Nitric Oxide System in Peripheral Artery Disease: Connection with Oxidative Stress and Biopterins.Antioxidants (Basel). 2020 Jul 6;9(7):590. doi: 10.3390/antiox9070590. Antioxidants (Basel). 2020. PMID: 32640613 Free PMC article.
-
Oxidative Stress in Peripheral Arterial Disease (PAD) Mechanism and Biomarkers.Antioxidants (Basel). 2019 Sep 2;8(9):367. doi: 10.3390/antiox8090367. Antioxidants (Basel). 2019. PMID: 31480714 Free PMC article. Review.
-
Associations of Obesity With Incident Hospitalization Related to Peripheral Artery Disease and Critical Limb Ischemia in the ARIC Study.J Am Heart Assoc. 2018 Aug 21;7(16):e008644. doi: 10.1161/JAHA.118.008644. J Am Heart Assoc. 2018. PMID: 30369315 Free PMC article.
References
-
- Chen A.F., Chen D.D., Daiber A., Faraci F.M., Li H., Rembold C.M., Laher I. Free radical biology of the cardiovascular system. Clin. Sci. 2012;123:73–91. - PubMed
-
- Yorek M.A. The role of oxidative stress in diabetic vascular and neural disease. Free Radic. Res. 2003;37:471–480. - PubMed
-
- Rossig L., Fichtlscherer B., Breitschopf K., Haendeler J., Zeiher A.M., Mulsch A., Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J. Biol. Chem. 1999;274:6823–6826. - PubMed
-
- Ullrich V., Kissner R. Redox signaling: bioinorganic chemistry at its best. J. Inorg. Biochem. 2006;100:2079–2086. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

