Inhibition of semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 reduces lipopolysaccharide-induced neuroinflammation
- PMID: 28437839
- PMCID: PMC5481640
- DOI: 10.1111/bph.13832
Inhibition of semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 reduces lipopolysaccharide-induced neuroinflammation
Abstract
Background and purpose: Neuroinflammation is initiated by a variety of stimuli including infections, sepsis, neurodegenerative diseases or traumatic brain injury and, if not adequately controlled, can lead to various degrees of neuronal damage and behavioural impairment. A critical event in the initial steps of inflammation is neutrophil extravasation. Semicarbazide-sensitive amine oxidase (SSAO, also known as vascular adhesion protein 1 or VAP-1) regulates neutrophil adhesion and extravasation. Here, we elucidate the role of SSAO/VAP-1 in the early stage inflammatory response after LPS insult in the brain.
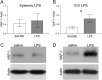
Experimental approach: PXS-4681A, a selective and irreversible SSAO/VAP-1 inhibitor, was tested in two rat models of neuroinflammation, following systemic or i.c.v. LPS. Immunohistochemical and immunofluorescence techniques were used to measure neutrophils and microglia. VAP-1 was quantitated by Western blotting.
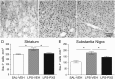
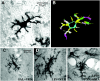
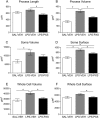
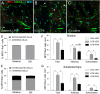
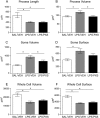
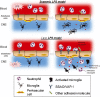
Key results: Both systemic and i.c.v. administration of LPS induced an increase in neutrophil recruitment and microglial response in various brain areas including the substantia nigra and striatum. PXS-4681A produced a significant inhibition of neutrophil recruitment and extravasation after i.c.v. LPS injection and also reversed microglial cell recruitment and morphological changes to the level of the sham controls in both LPS models.
Conclusions and implications: PXS-4681A acted as an effective anti-inflammatory agent after both systemic and i.c.v. LPS injections suggesting that SSAO/VAP-1 inhibition could be beneficial in the treatment of brain inflammation.
© 2017 The British Pharmacological Society.
Figures



Similar articles
-
Inhibition of vascular adhesion protein 1 protects dopamine neurons from the effects of acute inflammation and restores habit learning in the striatum.J Neuroinflammation. 2021 Oct 15;18(1):233. doi: 10.1186/s12974-021-02288-8. J Neuroinflammation. 2021. PMID: 34654450 Free PMC article.
-
PXS-4681A, a potent and selective mechanism-based inhibitor of SSAO/VAP-1 with anti-inflammatory effects in vivo.J Pharmacol Exp Ther. 2013 Nov;347(2):365-74. doi: 10.1124/jpet.113.207613. Epub 2013 Aug 13. J Pharmacol Exp Ther. 2013. PMID: 23943052
-
Effects of an anti-inflammatory VAP-1/SSAO inhibitor, PXS-4728A, on pulmonary neutrophil migration.Respir Res. 2015 Mar 20;16(1):42. doi: 10.1186/s12931-015-0200-z. Respir Res. 2015. PMID: 25889951 Free PMC article.
-
Semicarbazide-sensitive amine oxidase/vascular adhesion protein-1: a patent survey.Expert Opin Ther Pat. 2011 Sep;21(9):1453-71. doi: 10.1517/13543776.2011.594040. Epub 2011 Jun 15. Expert Opin Ther Pat. 2011. PMID: 21675926 Review.
-
Vascular adhesion protein-1 (VAP-1) in vascular inflammatory diseases.Vasa. 2022 Nov;51(6):341-350. doi: 10.1024/0301-1526/a001031. Epub 2022 Oct 6. Vasa. 2022. PMID: 36200383 Review.
Cited by
-
Human Copper-Containing Amine Oxidases in Drug Design and Development.Molecules. 2020 Mar 12;25(6):1293. doi: 10.3390/molecules25061293. Molecules. 2020. PMID: 32178384 Free PMC article. Review.
-
Sestrin2 provides cerebral protection through activation of Nrf2 signaling in microglia following subarachnoid hemorrhage.Front Immunol. 2023 Jan 24;14:1089576. doi: 10.3389/fimmu.2023.1089576. eCollection 2023. Front Immunol. 2023. PMID: 36761756 Free PMC article.
-
SSAO/VAP-1 in Cerebrovascular Disorders: A Potential Therapeutic Target for Stroke and Alzheimer's Disease.Int J Mol Sci. 2021 Mar 25;22(7):3365. doi: 10.3390/ijms22073365. Int J Mol Sci. 2021. PMID: 33805974 Free PMC article. Review.
-
The role of VAP-1 in cardiovascular disease: a review.Front Cardiovasc Med. 2025 May 19;12:1549157. doi: 10.3389/fcvm.2025.1549157. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40458597 Free PMC article. Review.
-
Development of a HPLC-FL method to determine benzaldehyde after derivatization with N-acetylhydrazine acridone and its application for determination of semicarbazide-sensitive amine oxidase activity in human serum.RSC Adv. 2019 Feb 26;9(12):6717-6723. doi: 10.1039/c8ra10004g. eCollection 2019 Feb 22. RSC Adv. 2019. PMID: 35518507 Free PMC article.
References
-
- Abella A, García‐Vicente S, Viguerie N, Ros‐Baró A, Camps M, Palacín M et al. (2004). Adipocytes release a soluble form of VAP‐1/SSAO by a metalloprotease‐dependent process and in a regulated manner. Diabetologia 47: 429–438. - PubMed
-
- Andonegui G, Goyert SM, Kubes P (2002). Lipopolysaccharide‐induced leukocyte‐endothelial cell interactions: a role for CD14 versus toll‐like receptor 4 within microvessels. J Immunol 169: 2111–2119. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

