Insights into the availability and distribution of oral artemisinin monotherapy in Myanmar: evidence from a nationally representative outlet survey
- PMID: 28438145
- PMCID: PMC5404336
- DOI: 10.1186/s12936-017-1793-0
Insights into the availability and distribution of oral artemisinin monotherapy in Myanmar: evidence from a nationally representative outlet survey
Abstract
Background: The containment of artemisinin resistance in Myanmar, historically an important probable origin and route of anti-malarial resistance to the India sub-continent and beyond, is crucial to global malaria control and elimination. This paper describes what is currently known about the sale and distribution of oral artemisinin monotherapy (AMT) across Myanmar, where this medicine is commonly found.
Methods: A nationally representative 2015 outlet survey was conducted in the private sector, and among community health workers across four geographical domains. A national sample of outlets was screened for availability of malaria testing and treatment, and an audit was completed for all anti-malarials.
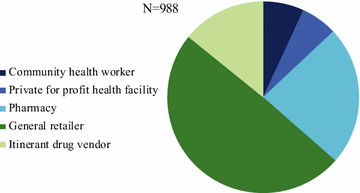
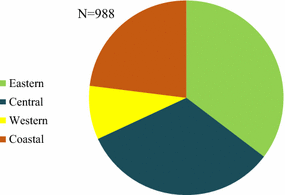
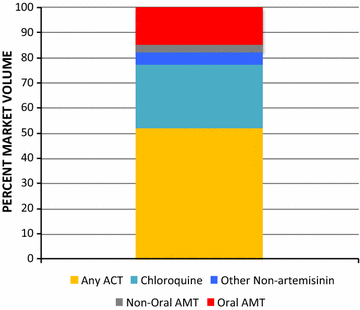
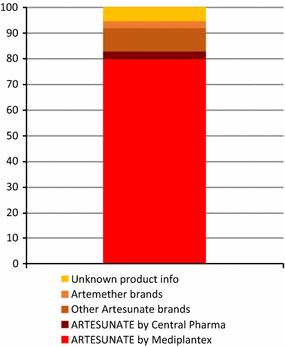
Results: A total of 3859 outlets across Myanmar had an anti-malarial in stock on the day of survey. Of the 3859 anti-malarial stocking outlets, 988 outlets stocked oral AMT. Availability of oral AMT was highest among outlets in the Western border (36.8%) versus other domains (Eastern, 15.0%; Central, 19.3% Coastal, 10.7%). Over 90% of the oral AMT service delivery points were private sector outlets: general retailers (49.4%), pharmacies (23.5%), and itinerant drug vendors (14.2%). Eleven unique oral AMT products were audited. The most common product audited was Artesunate®, manufactured by Mediplantex in Vietnam, which accounted for 79.9% of the oral AMT market share. Other oral AMT products were manufactured in China and in Myanmar. Over 60% of oral AMT products had a shelf life at purchase of greater than 2 years and only 14.7% were expired. The median number of oral AMT tablets typically dispensed to treat malaria was two tablets, approximately one tenth of a full adult course. The median price of a 50 mg tablet was $0.16.
Conclusions: Given the high availability and distribution of oral AMT, it is possible that Myanmar has become the last remaining viable market for any oral AMT in the region for manufacturers. National and international organizations need to act quickly and effectively to stop the production and distribution to both improve malaria control within Myanmar and reduce risk of artemisinin resistance spreading to India and Africa.
Figures
References
-
- WHO. Guidelines for the treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015. http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf. Accessed 28 Mar 2017.
-
- WHO. Emergence and spread of artemisinin resistance calls for intensified efforts to withdraw oral artemisinin monotherapy from the market. Geneva: World Health Organization; 2014. http://www.who.int/malaria/publications/atoz/oral-artemisinin-based-mono.... Accessed 28 Mar 2017.
-
- World Health Assembly Mandate 60.18, 2007. http://apps.who.int/gb/ebwha/pdf_files/WHASSA_WHA60-Rec1/E/cover-intro-6.... Accessed 28 Mar 2017.
-
- WHO. Marketing of oral artemisinin-based monotherapy medicines. Positions expressed by manufacturers. Geneva: World Health Organization; 2015. http://www.who.int/malaria/monotherapy_manufacturers.pdf?ua=1. Accessed 28 March 2017.
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical