A cluster randomised control trial to evaluate the effectiveness and cost-effectiveness of the Italian medicines use review (I-MUR) for asthma patients
- PMID: 28438152
- PMCID: PMC5404667
- DOI: 10.1186/s12913-017-2245-9
A cluster randomised control trial to evaluate the effectiveness and cost-effectiveness of the Italian medicines use review (I-MUR) for asthma patients
Abstract
Background: The economic burden of asthma, which relates to the degree of control, is €5 billion annually in Italy. Pharmacists could help improve asthma control, reducing this burden. This study aimed to evaluate the effectiveness and cost-effectiveness of Medicines Use Reviews provided by community pharmacists in asthma.
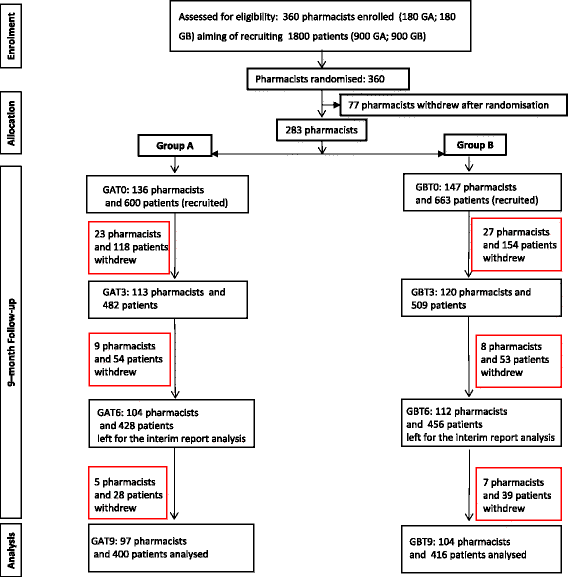
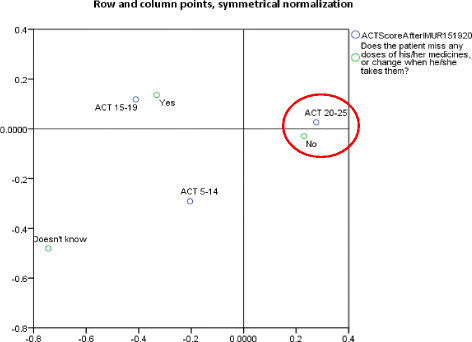
Methods: This cluster randomised, multi-centre, controlled trial in adult patients with asthma was conducted in 15 of the 20 regions of Italy between September 2014 and July 2015. After stratification by region, community pharmacists were randomly allocated to group A (trained in and delivered the intervention at baseline) or B (training and delivery 3 months later), using computerised random number generation in blocks of 10. Each recruited up to five patients, with both groups followed for 9 months. The intervention consisted of a systematic, structured face-to-face consultation with a pharmacist, covering asthma symptoms, medicines used, attitude towards medicines and adherence, recording pharmacist-identified pharmaceutical care issues (PCIs). The primary outcome was asthma control, assessed using the Asthma-Control-Test (ACT) score (ACT ≥ 20 represents good control). Secondary outcomes were: number of active ingredients, adherence, cost-effectiveness compared with usual care. Although blinding was not possible for either pharmacists or patients, assessment of outcomes was conducted by researchers blind to group allocation.
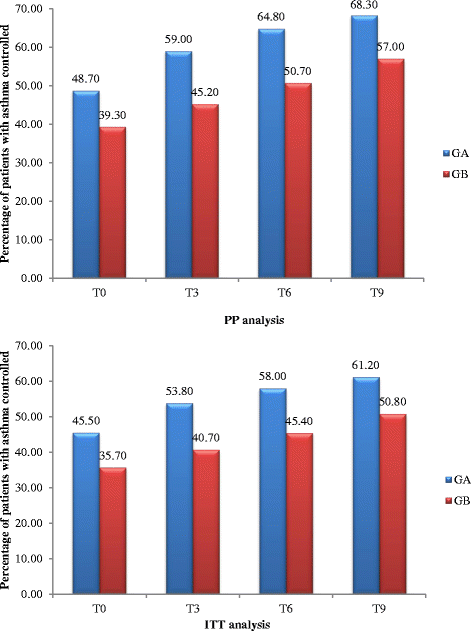
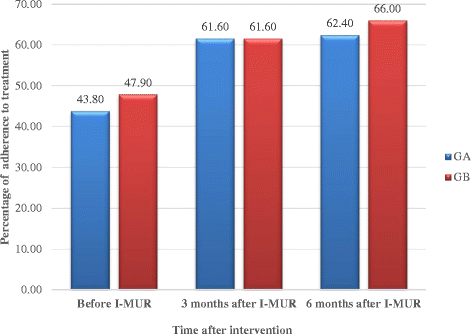
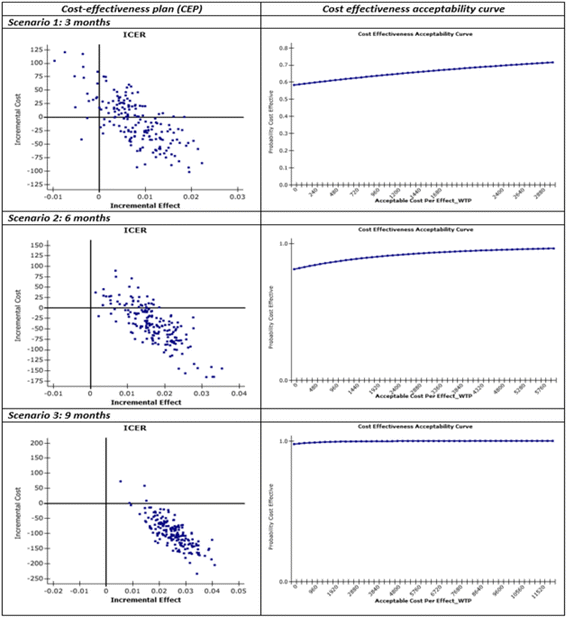
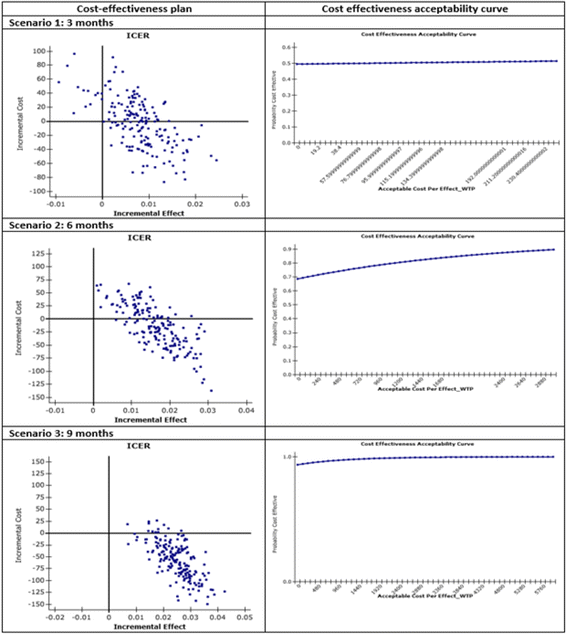
Results: Numbers of pharmacists and patients enrolled were 283 (A = 136; B = 147) and 1263 (A = 600; B = 663), numbers completing were 201 (A = 97; B = 104) and 816 (A = 400; B = 416), respectively. Patients were similar in age and gender and 56.13% (458/816) had poor/partial asthma control. Pharmacists identified 1256 PCIs (mean 1.54/patient), mostly need for education, monitoring and potentially ineffective therapy. Median ACT score at baseline differed between groups (A = 19, B = 18; p < 0.01). Odds ratio for improved asthma control was 1.76 (95% CI 1.33-2.33) and number needed to treat 10 (95% CI 6-28). Number of active ingredients reduced by 7.9% post-intervention (p < 0.01). Adherence improved by 35.4% 3 months post-intervention and 40.0% at 6 months (p < 0.01). The probability of the intervention being more cost-effective than usual care was 100% at 9 months.
Conclusions: This community pharmacist-based intervention demonstrated both effectiveness and cost-effectiveness. It has since been implemented as the first community pharmacy cognitive service in Italy.
Trial registration: TRN: ISRCTN72438848 (registered 5th January 2015, retrospectively).
Keywords: Asthma control; Community pharmacy; Cost-effectiveness; Effectiveness; Medicines use review.
Figures
References
-
- Masoli M, Fabian D, Holt S, Beasley R. Global Burden of Asthma. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-78. http://ginasthma.org/local/uploads/files/GINABurdenSummary_1.pdf (2003). Accessed 20 Oct 2015. - PubMed
-
- European Respiratory Society. European Lung White book. The economic burden of lung disease. http://www.erswhitebook.org/chapters/the-economic-burden-of-lung-disease/ (2016). Accessed 10 Sept 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

