Efficient vitrification of mouse embryos using the Kitasato Vitrification System as a novel vitrification device
- PMID: 28438181
- PMCID: PMC5404289
- DOI: 10.1186/s12958-017-0249-2
Efficient vitrification of mouse embryos using the Kitasato Vitrification System as a novel vitrification device
Abstract
Background: Currently, the cryopreservation of embryos and oocytes is essential for assisted reproductive technology (ART) laboratories worldwide. This study aimed to evaluate the efficacy of the Kitasato Vitrification System (KVS) as a vitrification device for the cryopreservation of mouse embryos to determine whether this novel device can be adapted to the field of ART.
Methods: In Experiment 1, blastocysts were vitrified using the KVS. Vitrified blastocysts were warmed and subsequently cultured for 72 h. In Experiment 2, 2-cell-stage embryos were vitrified using the KVS, and vitrified embryos were warmed and subsequently cultured for 96 h. In Experiment 3, we evaluated the in vivo developmental potential of vitrified 2-cell-stage embryos using the KVS, and in Experiment 4, we evaluated the cooling and warming rates for these devices using a numerical simulation.
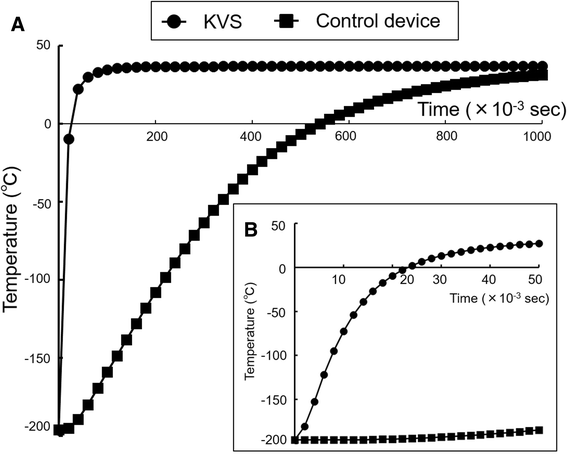
Results: In Experiment 1, there were no significant differences between the survival rates of the KVS and a control device. However, re-expanded (100%) and hatching (91.8%) rates were significantly higher for blastocysts vitrified using the KVS. In Experiment 2, there were no significant differences between the survival rates, or rates of development to the blastocyst stage, of vitrified and fresh embryos. In Experiment 3, after embryo transfer, 41% of the embryos developed into live offspring. In Experiment 4, the cooling and warming rates of the KVS were 683,000 and 612,000 °C/min, respectively, exceeding those of the control device.
Conclusions: Our study clearly demonstrates that the KVS is a novel vitrification device for the cryopreservation of mouse embryos at the blastocyst and 2-cell stage.
Keywords: Cryopreservation; Embryos; KVS; Ultra-rapid cooling; Vitrification.
Figures


Similar articles
-
A new vitrification device that absorbs excess vitrification solution adaptable to a closed system for the cryopreservation of mouse embryos.Cryobiology. 2019 Jun;88:9-14. doi: 10.1016/j.cryobiol.2019.04.008. Epub 2019 Apr 26. Cryobiology. 2019. PMID: 31034811
-
Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development.Hum Reprod. 2016 Aug;31(8):1685-95. doi: 10.1093/humrep/dew127. Epub 2016 Jun 6. Hum Reprod. 2016. PMID: 27270972
-
Preclinical validation of the new vitrification device possessing a feature of absorbing excess vitrification solution for the cryopreservation of human embryos.J Obstet Gynaecol Res. 2020 Feb;46(2):302-309. doi: 10.1111/jog.14176. Epub 2020 Jan 10. J Obstet Gynaecol Res. 2020. PMID: 31922309
-
A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos.Hum Reprod Update. 2012 Sep-Oct;18(5):536-54. doi: 10.1093/humupd/dms016. Epub 2012 Apr 25. Hum Reprod Update. 2012. PMID: 22537859 Review.
-
Recent developments in human oocyte, embryo and blastocyst vitrification: where are we now?Reprod Biomed Online. 2003 Dec;7(6):623-33. doi: 10.1016/s1472-6483(10)62084-6. Reprod Biomed Online. 2003. PMID: 14748959 Review.
Cited by
-
Alterations in Gene Expression and the Fatty Acid Profile Impact but Do Not Compromise the In Vitro Maturation of Zebrafish (Danio rerio) Stage III Ovarian Follicles after Cryopreservation.Animals (Basel). 2023 Nov 18;13(22):3563. doi: 10.3390/ani13223563. Animals (Basel). 2023. PMID: 38003179 Free PMC article.
-
A comprehensive review and update on human fertility cryopreservation methods and tools.Front Vet Sci. 2023 Apr 18;10:1151254. doi: 10.3389/fvets.2023.1151254. eCollection 2023. Front Vet Sci. 2023. PMID: 37143497 Free PMC article. Review.
-
An all-37 °C thawing method improves the clinical outcomes of vitrified frozen-thawed embryo transfer: a retrospective study using a case-control matching analysis.Arch Gynecol Obstet. 2023 Jun;307(6):1991-1999. doi: 10.1007/s00404-023-07029-1. Epub 2023 Apr 12. Arch Gynecol Obstet. 2023. PMID: 37041370
-
A human-based assisted reproduction protocol for the menstruating spiny mouse, Acomys cahirinus.PLoS One. 2020 Dec 28;15(12):e0244411. doi: 10.1371/journal.pone.0244411. eCollection 2020. PLoS One. 2020. PMID: 33370773 Free PMC article.
-
Cryodevices developed for minimum volume cooling vitrification of bovine oocytes.Anim Sci J. 2022 Jan-Dec;93(1):e13683. doi: 10.1111/asj.13683. Anim Sci J. 2022. PMID: 35075717 Free PMC article. Review.
References
-
- Kuwayama M, Kato O. All-round vitrification method for human oocytes and embryos. J Assist Reprod Genet. 2000;17:47.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

