Semaphorin-Plexin signaling influences early ventral telencephalic development and thalamocortical axon guidance
- PMID: 28438183
- PMCID: PMC5402653
- DOI: 10.1186/s13064-017-0083-4
Semaphorin-Plexin signaling influences early ventral telencephalic development and thalamocortical axon guidance
Abstract
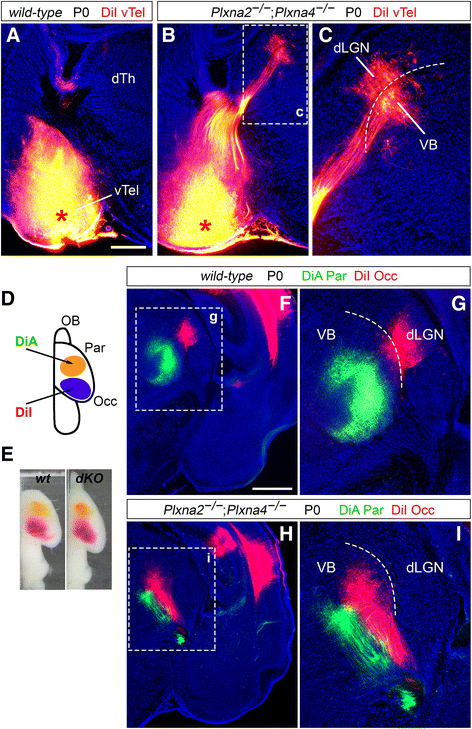
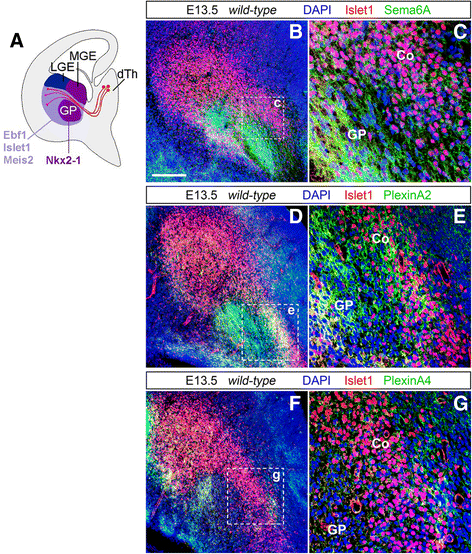
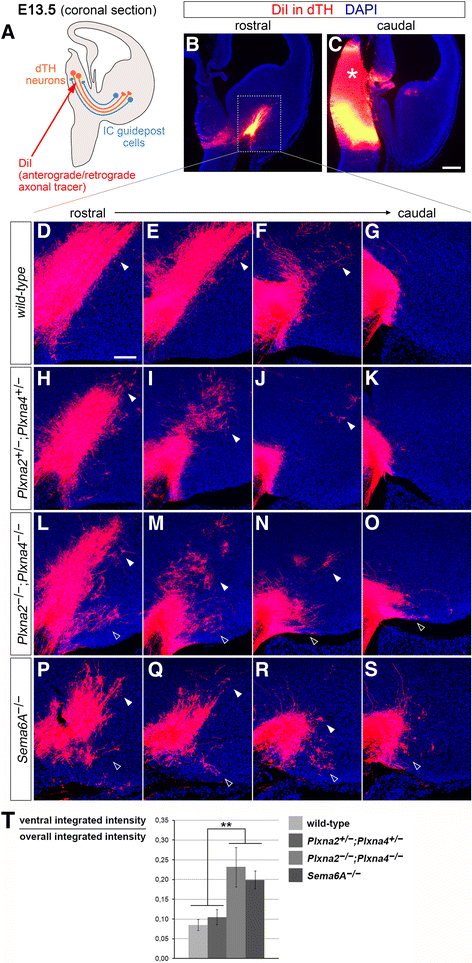
Background: Sensory processing relies on projections from the thalamus to the neocortex being established during development. Information from different sensory modalities reaching the thalamus is segregated into specialized nuclei, whose neurons then send inputs to cognate cortical areas through topographically defined axonal connections. Developing thalamocortical axons (TCAs) normally approach the cortex by extending through the subpallium; here, axonal navigation is aided by distributed guidance cues and discrete cell populations, such as the corridor neurons and the internal capsule (IC) guidepost cells. In mice lacking Semaphorin-6A, axons from the dorsal lateral geniculate nucleus (dLGN) bypass the IC and extend aberrantly in the ventral subpallium. The functions normally mediated by Semaphorin-6A in this system remain unknown, but might depend on interactions with Plexin-A2 and Plexin-A4, which have been implicated in other neurodevelopmental processes.
Methods: We performed immunohistochemical and neuroanatomical analyses of thalamocortical wiring and subpallial development in Sema6a and Plxna2; Plxna4 null mutant mice and analyzed the expression of these genes in relevant structures.
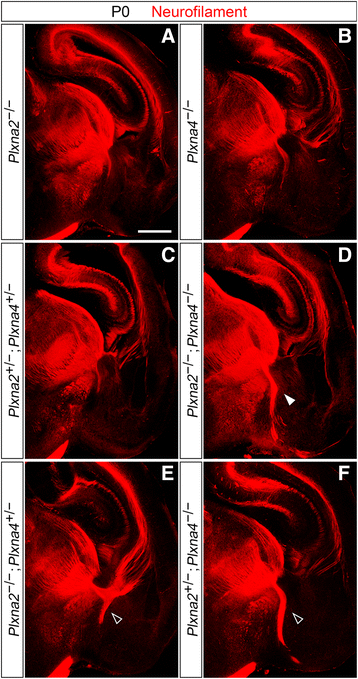
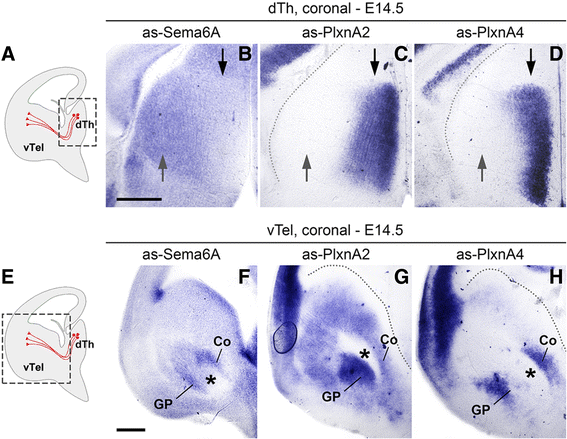
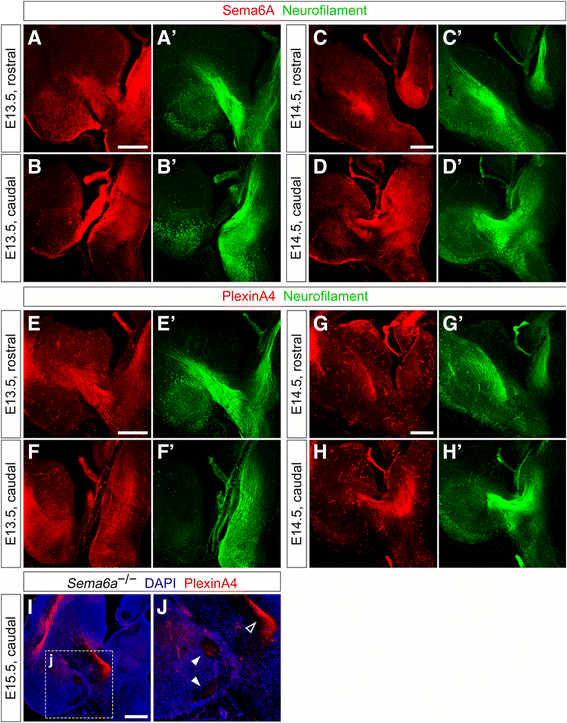
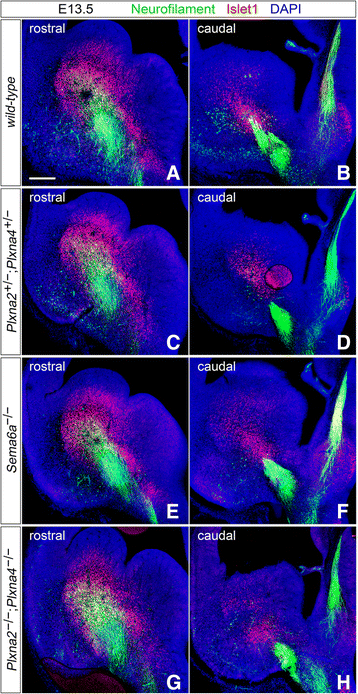
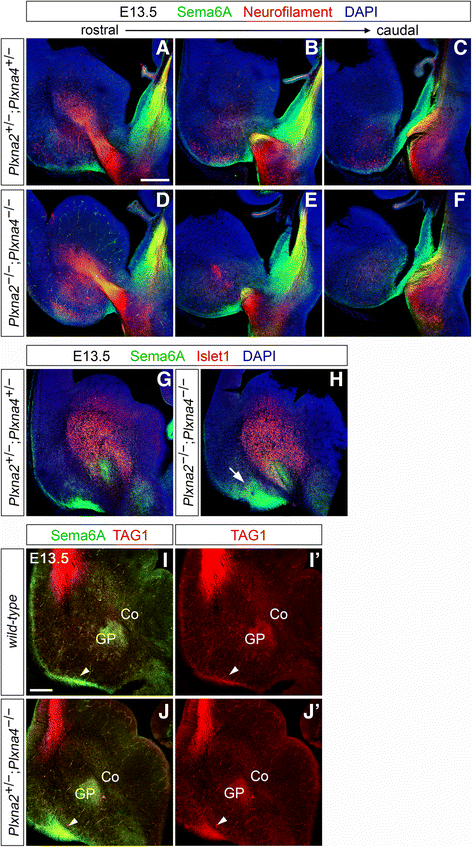
Results: In Plxna2; Plxna4 double mutants we discovered TCA pathfinding defects that mirrored those observed in Sema6a mutants, suggesting that Semaphorin-6A - Plexin-A2/Plexin-A4 signaling might mediate dLGN axon guidance at subpallial level. In order to understand where and when Semaphorin-6A, Plexin-A2 and Plexin-A4 may be required for proper subpallial TCA guidance, we then characterized their spatiotemporal expression dynamics during early TCA development. We observed that the thalamic neurons whose axons are misrouted in these mutants normally express Semaphorin-6A but not Plexin-A2 or Plexin-A4. By contrast, all three proteins are expressed in corridor cells and other structures in the developing basal ganglia. This finding could be consistent with an hypothetical action of Plexins as guidance signals through Sema6A as a receptor on dLGN axons, and/or with their indirect effect on TCA guidance due to functions in the morphogenesis of subpallial intermediate targets. In support of the latter possibility, we observed that in both Plxna2; Plxna4 and Sema6a mutants some IC guidepost cells abnormally localize in correspondence of the ventral path misrouted TCAs elongate into.
Conclusions: These findings implicate Semaphorin-6A - Plexin-A2/Plexin-A4 interactions in dLGN axon guidance and in the spatiotemporal organization of guidepost cell populations in the mammalian subpallium.
Keywords: Guidepost cells; Plexin-A2; Plexin-A4; Semaphorin-6A; Subpallium; Thalamocortical connectivity.
Figures
Similar articles
-
Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points.Neural Dev. 2008 Dec 8;3:34. doi: 10.1186/1749-8104-3-34. Neural Dev. 2008. PMID: 19063725 Free PMC article.
-
Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance.J Neurosci. 2005 Apr 6;25(14):3628-37. doi: 10.1523/JNEUROSCI.4480-04.2005. J Neurosci. 2005. PMID: 15814794 Free PMC article.
-
Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling.Neural Dev. 2008 Aug 26;3:21. doi: 10.1186/1749-8104-3-21. Neural Dev. 2008. PMID: 18727829 Free PMC article.
-
Molecular basis of semaphorin-mediated axon guidance.J Neurobiol. 2000 Aug;44(2):219-29. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. J Neurobiol. 2000. PMID: 10934324 Review.
-
Inputs from the thalamocortical system on axon pathfinding mechanisms.Curr Opin Neurobiol. 2014 Aug;27:143-50. doi: 10.1016/j.conb.2014.03.013. Epub 2014 Apr 17. Curr Opin Neurobiol. 2014. PMID: 24742382 Review.
Cited by
-
Genetics of physiological dysregulation: findings from the long life family study using joint models.Aging (Albany NY). 2020 Apr 1;12(7):5920-5947. doi: 10.18632/aging.102987. Epub 2020 Apr 1. Aging (Albany NY). 2020. PMID: 32235003 Free PMC article.
-
Transcriptome Profiling of Primary Skin Fibroblasts Reveal Distinct Molecular Features Between PLOD1- and FKBP14-Kyphoscoliotic Ehlers-Danlos Syndrome.Genes (Basel). 2019 Jul 8;10(7):517. doi: 10.3390/genes10070517. Genes (Basel). 2019. PMID: 31288483 Free PMC article.
-
AKT Signaling Modifies the Balance between Cell Proliferation and Migration in Neural Crest Cells from Patients Affected with Bosma Arhinia and Microphthalmia Syndrome.Biomedicines. 2021 Jun 29;9(7):751. doi: 10.3390/biomedicines9070751. Biomedicines. 2021. PMID: 34209568 Free PMC article.
-
The maternal microbiome modulates fetal neurodevelopment in mice.Nature. 2020 Oct;586(7828):281-286. doi: 10.1038/s41586-020-2745-3. Epub 2020 Sep 23. Nature. 2020. PMID: 32968276 Free PMC article.
-
Semaphorin 6A-Plexin A2/A4 Interactions with Radial Glia Regulate Migration Termination of Superficial Layer Cortical Neurons.iScience. 2019 Nov 22;21:359-374. doi: 10.1016/j.isci.2019.10.034. Epub 2019 Oct 21. iScience. 2019. PMID: 31698249 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

