Very small embryonic-like stem cells (VSELs) in adult mouse uterine perimetrium and myometrium
- PMID: 28438190
- PMCID: PMC5404303
- DOI: 10.1186/s13048-017-0324-5
Very small embryonic-like stem cells (VSELs) in adult mouse uterine perimetrium and myometrium
Abstract
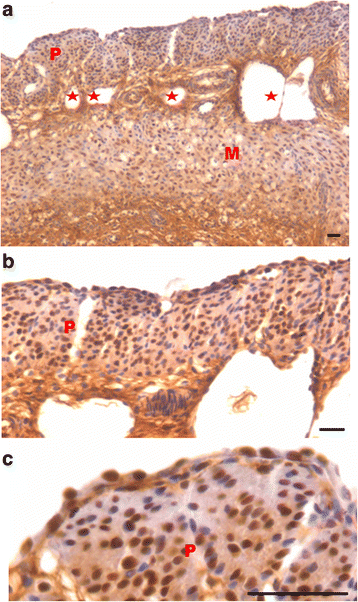
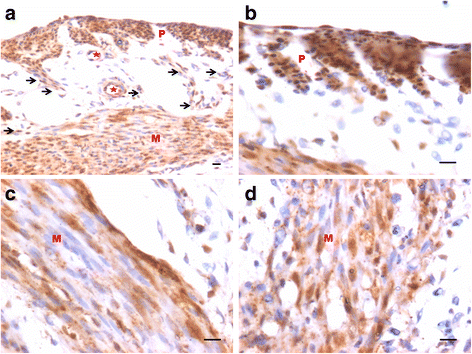
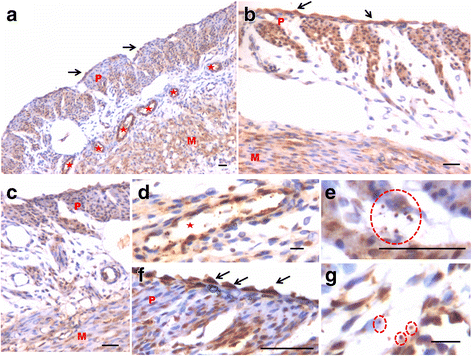
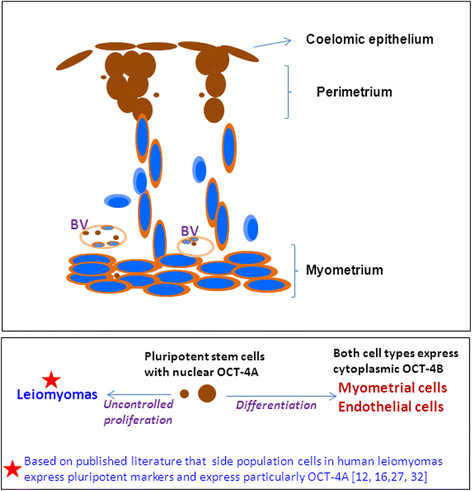
We have earlier reported the presence of very small embryonic-like stem cells (VSELs) in adult mouse uterus along with slightly bigger progenitors termed endometrial stem cells (EnSCs) and their regulation by ovarian hormones thus demonstrating a crucial role played by them during proliferation, differentiation and remodeling of the endometrium. Present study is a brief communication wherein we have examined the effect of higher dose of estrogen (E, 2 μg/day), progesterone (P, 1 mg/day) and follicle stimulating hormone (FSH, 5 IU/day for 5 days) specifically on the myometrium and perimetrium surrounding the endometrium in bilaterally ovariectomized mice. Similar treatment with E & P was recently used in a study published in the journal Nature to study the effect of steroid hormones on hematopoietic stem cells and this treatment regimen helps achieve hormone levels observed during pregnancy. Quiescent spherical stem cells (lacking PCNA expression) with high nucleo-cytoplasmic ratio and nuclear OCT-4A were detected in the perimetrium of atrophied (bilaterally ovariectomized) uterus. PCNA expression was observed after treatment and cells with cytoplasmic OCT-4B were invariably observed in the myometrium. VSELs were clearly visualized after treatment and the effect of P and FSH was more prominent compared to E on the development of myometrium. It is speculated that stem cells with nuclear OCT-4A located in the perimetrium differentiate to give rise to endothelial and myometrial cells with cytoplasmic OCT-4B. Based on the results of present study and published reports showing the presence of pluripotent markers (OCT-4, NANOG and SOX2) in human myometrial side population and expression of particularly OCT-4A in human leiomyomas, we speculate that these nuclear OCT-4 positive stem cells located in the perimetrium are the possible tumor initiating cells leading to the development of leiomyomas rather than the mesenchymal cells which express cytoplasmic OCT-4B.
Keywords: Hormones; Leiomyomas; Myometrium; Uterus; VSELs.
Figures









Similar articles
-
Endogenous, tissue-resident stem/progenitor cells in gonads and bone marrow express FSHR and respond to FSH via FSHR-3.J Ovarian Res. 2021 Oct 30;14(1):145. doi: 10.1186/s13048-021-00883-0. J Ovarian Res. 2021. PMID: 34717703 Free PMC article. Review.
-
Gonadotropin and steroid hormones regulate pluripotent very small embryonic-like stem cells in adult mouse uterine endometrium.J Ovarian Res. 2018 Sep 21;11(1):83. doi: 10.1186/s13048-018-0454-4. J Ovarian Res. 2018. PMID: 30241552 Free PMC article.
-
Very small embryonic-like stem cells are the elusive mouse endometrial stem cells--a pilot study.J Ovarian Res. 2015 Mar 11;8:9. doi: 10.1186/s13048-015-0138-2. J Ovarian Res. 2015. PMID: 25824685 Free PMC article.
-
Mice Uterine Stem Cells are Affected by Neonatal Endocrine Disruption & Initiate Uteropathies in Adult Life Independent of Circulatory Ovarian Hormones.Stem Cell Rev Rep. 2022 Jun;18(5):1686-1701. doi: 10.1007/s12015-021-10279-8. Epub 2021 Nov 9. Stem Cell Rev Rep. 2022. PMID: 34750780
-
Very small embryonic-like stem cells are involved in pancreatic regeneration and their dysfunction with age may lead to diabetes and cancer.Stem Cell Res Ther. 2015 May 15;6(1):96. doi: 10.1186/s13287-015-0084-3. Stem Cell Res Ther. 2015. PMID: 25976079 Free PMC article. Review.
Cited by
-
MicroRNAs regulate granulosa cells apoptosis and follicular development - A review.Asian-Australas J Anim Sci. 2020 Nov;33(11):1714-1724. doi: 10.5713/ajas.19.0707. Epub 2019 Dec 24. Asian-Australas J Anim Sci. 2020. PMID: 32054175 Free PMC article.
-
Additional evidence to support OCT-4 positive VSELs and EnSCs as the elusive tissue-resident stem/progenitor cells in adult mice uterus.Stem Cell Res Ther. 2022 Feb 5;13(1):60. doi: 10.1186/s13287-022-02703-8. Stem Cell Res Ther. 2022. PMID: 35123545 Free PMC article.
-
5-Azacytidine-Induced Cardiomyocyte Differentiation of Very Small Embryonic-Like Stem Cells.Stem Cells Int. 2020 Sep 8;2020:5162350. doi: 10.1155/2020/5162350. eCollection 2020. Stem Cells Int. 2020. PMID: 32963547 Free PMC article.
-
Changes in the Expression of Pluripotency Factor Oct-4 and Intensity of Apoptosis in the Uterus during Spontaneous and Immune-Dependent Abortions in Mice.Bull Exp Biol Med. 2022 Apr;172(6):765-769. doi: 10.1007/s10517-022-05474-7. Epub 2022 May 2. Bull Exp Biol Med. 2022. PMID: 35501657
-
Endogenous, tissue-resident stem/progenitor cells in gonads and bone marrow express FSHR and respond to FSH via FSHR-3.J Ovarian Res. 2021 Oct 30;14(1):145. doi: 10.1186/s13048-021-00883-0. J Ovarian Res. 2021. PMID: 34717703 Free PMC article. Review.
References
-
- Shaikh A, Anand S, Kapoor S, Ganguly R, Bhartiya D. Mouse bone marrow VSELs exhibit differentiation into three embryonic germ lineages and germ & hematopoietic cells in culture. Stem Cell Rev. 2017. doi: 10.1007/s12015-016-9714-0. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

