Extrastriatal changes in patients with late-onset glutaric aciduria type I highlight the risk of long-term neurotoxicity
- PMID: 28438223
- PMCID: PMC5402644
- DOI: 10.1186/s13023-017-0612-6
Extrastriatal changes in patients with late-onset glutaric aciduria type I highlight the risk of long-term neurotoxicity
Abstract
Background: Without neonatal initiation of treatment, 80-90% of patients with glutaric aciduria type 1 (GA1) develop striatal injury during the first six years of life resulting in a complex, predominantly dystonic movement disorder. Onset of motor symptoms may be acute following encephalopathic crisis or insidious without apparent crisis. Additionally, so-called late-onset GA1 has been described in single patients diagnosed after the age of 6 years. With the aim of better characterizing and understanding late-onset GA1 we analyzed clinical findings, biochemical phenotype, and MRI changes of eight late-onset patients and compared these to eight control patients over the age of 6 years with early diagnosis and start of treatment.
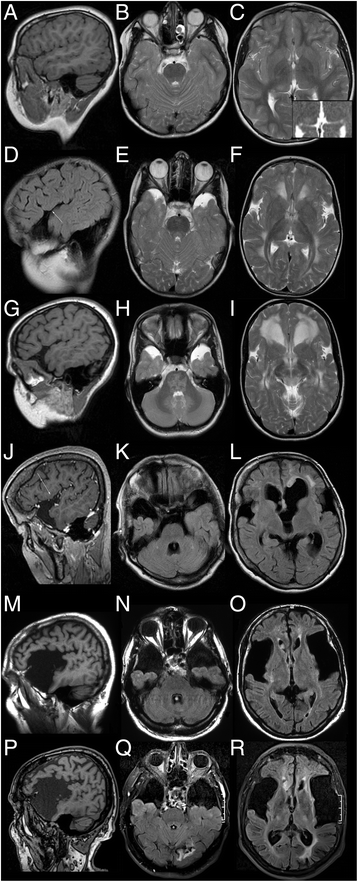
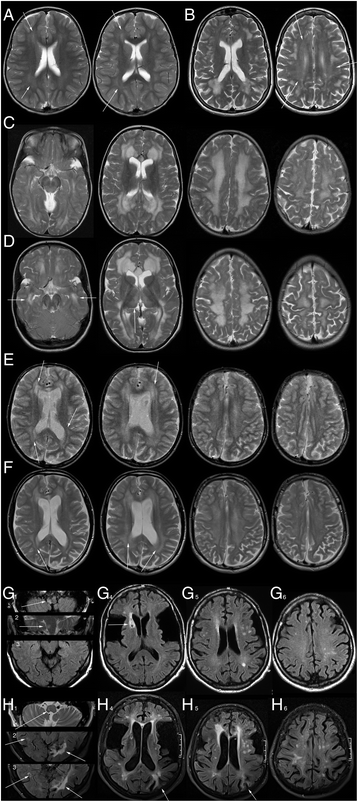
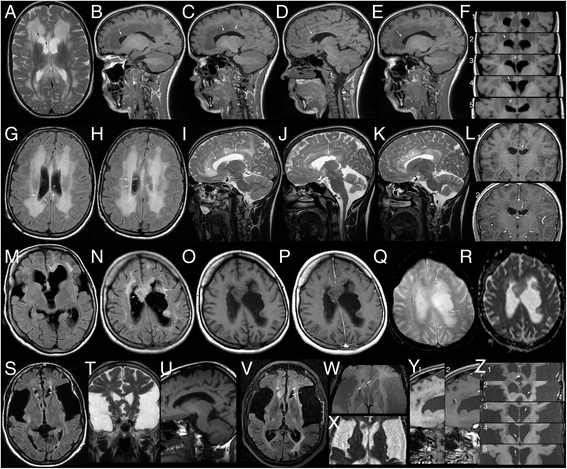
Results: No late-onset or control patient had either dystonia or striatal lesions on MRI. All late-onset (8/8) patients were high excretors, but only four of eight control patients. Two of eight late-onset patients were diagnosed after the age of 60 years, presenting with dementia, tremor, and epilepsy, while six were diagnosed before the age of 30 years: Three were asymptomatic mothers identified by following a positive screening result in their newborns and three had non-specific general symptoms, one with additional mild neurological deficits. Frontotemporal hypoplasia and white matter changes were present in all eight and subependymal lesions in six late-onset patients. At comparable age a greater proportion of late-onset patients had (non-specific) clinical symptoms and possibly subependymal nodules compared to control patients, in particular in comparison to the four clinically and MR-wise asymptomatic low-excreting control patients.
Conclusions: While clinical findings are non-specific, frontotemporal hypoplasia and subependymal nodules are characteristic MRI findings of late-onset GA1 and should trigger diagnostic investigation for this rare disease. Apart from their apparent non-susceptibility for striatal injury despite lack of treatment, patients with late-onset GA1 are not categorically different from early treated control patients. Differences between late-onset patients and early treated control patients most likely reflect greater cumulative neurotoxicity in individuals remaining undiagnosed and untreated for years, even decades as well as the higher long-term risk of high excretors for intracerebral accumulation of neurotoxic metabolites compared to low excretors.
Keywords: Frontotemporal hypoplasia; Glutaric aciduria type I; High excretor; Late-onset; Long-term disease course; Subependymal nodules.
Figures
Similar articles
-
Patterns, evolution, and severity of striatal injury in insidious- vs acute-onset glutaric aciduria type 1.J Inherit Metab Dis. 2019 Jan;42(1):117-127. doi: 10.1002/jimd.12033. J Inherit Metab Dis. 2019. PMID: 30740735
-
Adult-onset glutaric aciduria type I: rare presentation of a treatable disorder.Neurogenetics. 2020 Jul;21(3):179-186. doi: 10.1007/s10048-020-00610-9. Epub 2020 Apr 18. Neurogenetics. 2020. PMID: 32306145
-
(1)H-MRS in glutaric aciduria type 1: impact of biochemical phenotype and age on the cerebral accumulation of neurotoxic metabolites.J Inherit Metab Dis. 2015 Sep;38(5):829-38. doi: 10.1007/s10545-015-9826-8. Epub 2015 Apr 10. J Inherit Metab Dis. 2015. PMID: 25860816
-
Inherited Disorders of Lysine Metabolism: A Review.J Nutr. 2020 Oct 1;150(Suppl 1):2556S-2560S. doi: 10.1093/jn/nxaa112. J Nutr. 2020. PMID: 33000154 Review.
-
Impact of newborn screening and quality of therapy on the neurological outcome in glutaric aciduria type 1: a meta-analysis.Genet Med. 2021 Jan;23(1):13-21. doi: 10.1038/s41436-020-00971-4. Epub 2020 Sep 28. Genet Med. 2021. PMID: 32981931 Free PMC article.
Cited by
-
Inborn Errors of Metabolism in Adults: Two Patients with Movement Disorders Caused by Glutaric Aciduria Type 1.Mov Disord Clin Pract. 2020 Sep 29;7(Suppl 3):S85-S88. doi: 10.1002/mdc3.13054. eCollection 2020 Sep. Mov Disord Clin Pract. 2020. PMID: 33015233 Free PMC article. No abstract available.
-
Evaluation of the Clinical, Biochemical, Neurological, and Genetic Presentations of Glutaric Aciduria Type 1 in Patients From China.Front Genet. 2021 Jul 7;12:702374. doi: 10.3389/fgene.2021.702374. eCollection 2021. Front Genet. 2021. PMID: 34306040 Free PMC article.
-
Metabolic Serendipities of Expanded Newborn Screening.Genes (Basel). 2020 Aug 29;11(9):1018. doi: 10.3390/genes11091018. Genes (Basel). 2020. PMID: 32872442 Free PMC article.
-
Biochemical and molecular features of Chinese patients with glutaric acidemia type 1 detected through newborn screening.Orphanet J Rare Dis. 2021 Aug 3;16(1):339. doi: 10.1186/s13023-021-01964-5. Orphanet J Rare Dis. 2021. PMID: 34344405 Free PMC article.
-
Phenotypic and Genotypic Characteristics of Adult-Onset Glutaric Aciduria Type 1: Report of Two Cases and a Literature Review.Brain Behav. 2025 Feb;15(2):e70281. doi: 10.1002/brb3.70281. Brain Behav. 2025. PMID: 39963939 Free PMC article. Review.
References
-
- Baric I, Wagner L, Feyh P, Liesert M, Buckel W, Hoffmann G. Sensitivity and specificity of free and total glutaric and 3-hydroxyglutaric acid measurements of stable-sotope dilution assays for the diagnosis of glutaric aciduria type I. J Inherit Metab Dis. 1999;22:867–881. doi: 10.1023/A:1005683222187. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

