Applications of pHLIP Technology for Cancer Imaging and Therapy
- PMID: 28438340
- PMCID: PMC5492987
- DOI: 10.1016/j.tibtech.2017.03.014
Applications of pHLIP Technology for Cancer Imaging and Therapy
Erratum in
-
Applications of pHLIP Technology for Cancer Imaging and Therapy: (Trends in Biotechnology 35, 653-664, 2017).Trends Biotechnol. 2018 Dec;36(12):1300. doi: 10.1016/j.tibtech.2017.11.005. Epub 2017 Nov 30. Trends Biotechnol. 2018. PMID: 29198980 Free PMC article. No abstract available.
Abstract
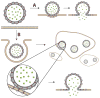
Acidity is a biomarker of cancer that is not subject to the blunting clonal selection effects that reduce the efficacy of other biomarker technologies, such as antibody targeting. The pH (low) insertion peptides (pHLIP®s) provide new opportunities for targeting acidic tissues. Through the physical mechanism of membrane-associated folding, pHLIPs are triggered by the acidic microenvironment to insert and span the membranes of tumor cells. The pHLIP platform can be applied to imaging acidic tissues, delivering cell-permeable and impermeable molecules to the cytoplasm, and promoting the cellular uptake of nanoparticles. Since acidosis is a hallmark of tumor development, progression, and aggressiveness, the pHLIP technology may prove useful in targeting cancer cells and metastases for tumor diagnosis, imaging, and therapy.
Keywords: acidity; drug delivery; imaging; nanotechnology; tumor targeting.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



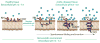
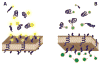
Similar articles
-
pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents.Mol Membr Biol. 2010 Oct;27(7):341-52. doi: 10.3109/09687688.2010.509285. Epub 2010 Oct 13. Mol Membr Biol. 2010. PMID: 20939768 Free PMC article. Review.
-
Targeting acidity in diseased tissues: mechanism and applications of the membrane-inserting peptide, pHLIP.Arch Biochem Biophys. 2015 Jan 1;565:40-8. doi: 10.1016/j.abb.2014.11.002. Epub 2014 Nov 18. Arch Biochem Biophys. 2015. PMID: 25444855 Free PMC article.
-
Advanced targeted nanomedicine.J Biotechnol. 2015 May 20;202:88-97. doi: 10.1016/j.jbiotec.2015.01.009. Epub 2015 Jan 20. J Biotechnol. 2015. PMID: 25615945 Free PMC article.
-
pHLIP Peptides Target Acidity in Activated Macrophages.Mol Imaging Biol. 2022 Dec;24(6):874-885. doi: 10.1007/s11307-022-01737-x. Epub 2022 May 23. Mol Imaging Biol. 2022. PMID: 35604527 Free PMC article.
-
Targeting diseased tissues by pHLIP insertion at low cell surface pH.Front Physiol. 2014 Mar 13;5:97. doi: 10.3389/fphys.2014.00097. eCollection 2014. Front Physiol. 2014. PMID: 24659971 Free PMC article. Review.
Cited by
-
Targeting the Hypoxic and Acidic Tumor Microenvironment with pH-Sensitive Peptides.Cells. 2021 Mar 4;10(3):541. doi: 10.3390/cells10030541. Cells. 2021. PMID: 33806273 Free PMC article. Review.
-
ES-MION-Based Dual-Modality PET/MRI Probes for Acidic Tumor Microenvironment Imaging.ACS Omega. 2022 Jan 18;7(4):3442-3451. doi: 10.1021/acsomega.1c05815. eCollection 2022 Feb 1. ACS Omega. 2022. PMID: 35128253 Free PMC article.
-
Selective Display of a Chemoattractant Agonist on Cancer Cells Activates the Formyl Peptide Receptor 1 on Immune Cells.Chembiochem. 2022 Apr 20;23(8):e202100521. doi: 10.1002/cbic.202100521. Epub 2022 Mar 9. Chembiochem. 2022. PMID: 35199442 Free PMC article.
-
Kinetics of pHLIP peptide insertion into and exit from a membrane.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12095-12100. doi: 10.1073/pnas.1917857117. Epub 2020 May 14. Proc Natl Acad Sci U S A. 2020. PMID: 32409607 Free PMC article.
-
Causes, consequences, and therapy of tumors acidosis.Cancer Metastasis Rev. 2019 Jun;38(1-2):205-222. doi: 10.1007/s10555-019-09792-7. Cancer Metastasis Rev. 2019. PMID: 30911978 Free PMC article. Review.
References
-
- Warburg O, et al. Über den Stoffwechsel der Tumoren. Biochem Z. 1924;152:319–344.
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Krebs HA. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. - PubMed
-
- Wykoff CC, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

