Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial
- PMID: 28438473
- PMCID: PMC5715461
- DOI: 10.1016/S1470-2045(17)30279-6
Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial
Abstract
Background: Platinum-based chemotherapy doublets are a standard of care for women with ovarian cancer recurring 6 months after completion of initial therapy. In this study, we aimed to explore the roles of secondary surgical cytoreduction and bevacizumab in this population, and report the results of the bevacizumab component here.
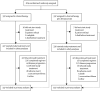
Methods: The multicentre, open-label, randomised phase 3 GOG-0213 trial was done in 67 predominantly academic centres in the USA (65 centres), Japan (one centre), and South Korea (one centre). Eligible patients were adult women (aged ≥18 years) with recurrent measurable or evaluable epithelial ovarian, primary peritoneal, or fallopian tube cancer, and a clinical complete response to primary platinum-based chemotherapy, who had been disease-free for at least 6 months following last infused cycle of platinum. Patients were randomly assigned (1:1) to standard chemotherapy (six 3-weekly cycles of paclitaxel [175 mg/m2 of body surface area] and carboplatin [area under the curve 5]) or the same chemotherapy regimen plus bevacizumab (15 mg/kg of bodyweight) every 3 weeks and continued as maintenance every 3 weeks until disease progression or unacceptable toxicity. Individuals who participated in both the bevacizumab objective and surgical objective (which is ongoing) were randomly assigned (1:1:1:1) to receive either of these two chemotherapy regimens with or without prior secondary cytoreductive surgery. Randomisation for the bevacizumab objective was stratified by treatment-free interval and participation in the surgical objective. The primary endpoint was overall survival, analysed by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00565851.
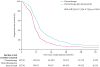
Findings: Between Dec 10, 2007, and Aug 26, 2011, 674 women were enrolled and randomly assigned to standard chemotherapy (n=337) or chemotherapy plus bevacizumab (n=377). Median follow-up at the end of the trial on Nov 5, 2014, was 49·6 months in each treatment group (IQR 41·5-62·2 for chemotherapy plus bevacizumab; IQR 40·8-59·3 for chemotherapy), at which point 415 patients had died (214 in the chemotherapy group and 201 in the chemotherapy plus bevacizumab group). Based on pretreatment stratification data, median overall survival in the chemotherapy plus bevacizumab group was 42·2 months (95% CI 37·7-46·2) versus 37·3 months (32·6-39·7) in the chemotherapy group (hazard ratio [HR] 0·829; 95% CI 0·683-1·005; p=0·056). We identified incorrect treatment-free interval stratification data for 45 (7%) patients (equally balanced between treatment groups); a sensitivity analysis of overall survival based on the audited treatment-free interval stratification data gave an adjusted HR of 0·823 (95% CI 0·680-0·996; p=0·0447). In the safety population (all patients who initiated treatment), 317 (96%) of 325 patients in the chemotherapy plus bevacizumab group had at least one grade 3 or worse adverse event compared with 282 (86%) of 332 in the chemotherapy group; the most frequently reported of these in the chemotherapy plus bevacizumab group compared with the chemotherapy group were hypertension (39 [12%] vs two [1%]), fatigue (27 [8%] vs eight [2%]), and proteinuria (27 [8%] vs none). Two (1%) treatment-related deaths occurred in the chemotherapy group (infection [n=1] and myelodysplastic syndrome [n=1]) compared with nine (3%) in the chemotherapy plus bevacizumab group (infection [n=1], febrile neutropenia [n=1], myelodysplastic syndrome [n=1], secondary malignancy [n=1]; deaths not classified with CTCAE terms: disease progression [n=3], sudden death [n=1], and not specified [n=1]).
Interpretation: The addition of bevacizumab to standard chemotherapy, followed by maintenance therapy until progression, improved the median overall survival in patients with platinum-sensitive recurrent ovarian cancer. Although the intention-to-treat analysis for overall survival was not significant, our sensitivity analysis based on corrected treatment-free interval stratification indicates that this strategy might be an important addition to the therapeutic armamentarium in these patients.
Funding: National Cancer Institute and Genentech.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
A new standard of care or just another option for patients with relapsed ovarian cancer?Lancet Oncol. 2017 Jun;18(6):701-702. doi: 10.1016/S1470-2045(17)30253-X. Epub 2017 Apr 21. Lancet Oncol. 2017. PMID: 28438475 No abstract available.
References
-
- Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. - PubMed
-
- Monk BJ, Coleman RL. Changing the paradigm in the treatment of platinum-sensitive recurrent ovarian cancer: from platinum doublets to nonplatinum doublets and adding antiangiogenesis compounds. Int J Gynecol Cancer. 2009;19(suppl 2):S63–67. - PubMed
-
- Schlaeppi JM, Eppenberger U, Martiny-Baron G, et al. Chemiluminescence immunoassay for vascular endothelial growth factor (vascular permeability factor) in tumor-tissue homogenates. Clin Chem. 1996;42:1777–84. - PubMed
-
- Ueda M, Hung YC, Terai Y, et al. Vascular endothelial growth factor-C expression and invasive phenotype in ovarian carcinomas. Clin Cancer Res. 11:3225–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- U10 CA180834/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- U10 CA027469/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U10 CA180798/CA/NCI NIH HHS/United States
- U10 CA037517/CA/NCI NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

