FDA Approval Summary: Nivolumab for the Treatment of Relapsed or Progressive Classical Hodgkin Lymphoma
- PMID: 28438889
- PMCID: PMC5423515
- DOI: 10.1634/theoncologist.2017-0004
FDA Approval Summary: Nivolumab for the Treatment of Relapsed or Progressive Classical Hodgkin Lymphoma
Abstract
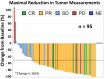
On May 17, 2016, after an expedited priority review, the U.S. Food and Drug Administration granted accelerated approval to nivolumab for the treatment of patients with classical Hodgkin lymphoma (cHL) that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and post-transplantation brentuximab vedotin (BV). Nivolumab in cHL had been granted breakthrough therapy designation. Accelerated approval was based on two single-arm, multicenter trials in adults with cHL. In 95 patients with relapsed or progressive cHL after autologous HSCT and post-transplantation BV, nivolumab, dosed at 3 mg/kg intravenously every 2 weeks, produced a 65% (95% confidence interval: 55%-75%) objective response rate (58% partial remission, 7% complete remission). The estimated median duration of response was 8.7 months, with 4.6-month median follow-up for response duration. The median time to response was 2.1 (range: 0.7-5.7) months. Among 263 patients with cHL treated with nivolumab, 21% reported serious adverse reactions (ARs). The most common all-grade ARs (reported in ≥20%) were fatigue, upper respiratory tract infection, cough, pyrexia, diarrhea, elevated transaminases, and cytopenias. Infusion-related reaction and hypothyroidism or thyroiditis occurred in >10% of patients; other immune-mediated ARs, occurring in 1%-5%, included rash, pneumonitis, hepatitis, hyperthyroidism, and colitis. A new Warning and Precaution was issued for complications of allogeneic HSCT after nivolumab, including severe or hyperacute graft-versus-host disease, other immune-mediated ARs, and transplant-related mortality. Continued approval for the cHL indication may be contingent upon verification of clinical benefit in a randomized trial. The Oncologist 2017;22:585-591 IMPLICATIONS FOR PRACTICE: Based on response rate and duration in single-arm studies, nivolumab is a new treatment option for patients with classical Hodgkin lymphoma (cHL) that has relapsed or progressed despite autologous hematopoietic stem cell transplantation (HSCT) and brentuximab vedotin. This was the first U.S. Food and Drug Administration marketing application for a programmed cell death 1 inhibitor in hematologic malignancies. The use of immune checkpoint blockade in cHL represents a new treatment paradigm. The safety of allogeneic HSCT after nivolumab requires further evaluation, as does the safety of nivolumab after allogeneic HSCT.
Keywords: Hodgkin lymphoma; Nivolumab; Programmed cell death 1 inhibitor; Programmed death‐ligand 1.
© AlphaMed Press 2017.
Figures
References
-
- Akpek G, Ambinder RF, Piantadosi S et al. Long‐term results of blood and marrow transplantation for Hodgkin's lymphoma. J Clin Oncol 2001;19:4314–4321. - PubMed
-
- Alinari L, Blum KA. How I treat relapsed classical Hodgkin lymphoma after autologous stem cell transplant. Blood 2016;127:287–295. - PubMed
-
- Burroughs LM, O'Donnell PV, Sandmaier BM et al. Comparison of outcomes of HLA‐matched related, unrelated, or HLA‐haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2008;14:1279–1287. - PMC - PubMed
-
- Chen R, Palmer JM, Popplewell L et al. Reduced intensity allogeneic hematopoietic cell transplantation can induce durable remission in heavily pretreated relapsed Hodgkin lymphoma. Ann Hematol 2011;90:803–808. - PubMed
-
- de Claro RA, McGinn K, Kwitkowski V et al. U.S. Food and Drug Administration approval summary: Brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large‐cell lymphoma. Clin Cancer Res 2012;18:5845–5849. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

