Interacting effects of land use and climate on rodent-borne pathogens in central Kenya
- PMID: 28438909
- PMCID: PMC5413868
- DOI: 10.1098/rstb.2016.0116
Interacting effects of land use and climate on rodent-borne pathogens in central Kenya
Abstract
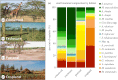
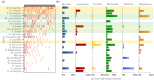
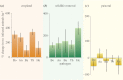
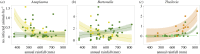
Understanding the effects of anthropogenic disturbance on zoonotic disease risk is both a critical conservation objective and a public health priority. Here, we evaluate the effects of multiple forms of anthropogenic disturbance across a precipitation gradient on the abundance of pathogen-infected small mammal hosts in a multi-host, multi-pathogen system in central Kenya. Our results suggest that conversion to cropland and wildlife loss alone drive systematic increases in rodent-borne pathogen prevalence, but that pastoral conversion has no such systematic effects. The effects are most likely explained both by changes in total small mammal abundance, and by changes in relative abundance of a few high-competence species, although changes in vector assemblages may also be involved. Several pathogens responded to interactions between disturbance type and climatic conditions, suggesting the potential for synergistic effects of anthropogenic disturbance and climate change on the distribution of disease risk. Overall, these results indicate that conservation can be an effective tool for reducing abundance of rodent-borne pathogens in some contexts (e.g. wildlife loss alone); however, given the strong variation in effects across disturbance types, pathogen taxa and environmental conditions, the use of conservation as public health interventions will need to be carefully tailored to specific pathogens and human contexts.This article is part of the themed issue 'Conservation, biodiversity and infectious disease: scientific evidence and policy implications'.
Keywords: dilution effect; disease; diversity; land-use change; landscape ecology; susceptible host regulation.
© 2017 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa.Proc Natl Acad Sci U S A. 2014 May 13;111(19):7036-41. doi: 10.1073/pnas.1404958111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778215 Free PMC article.
-
Conservation of biodiversity as a strategy for improving human health and well-being.Philos Trans R Soc Lond B Biol Sci. 2017 Jun 5;372(1722):20160131. doi: 10.1098/rstb.2016.0131. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28438920 Free PMC article. Review.
-
Land-use change and rodent-borne diseases: hazards on the shared socioeconomic pathways.Philos Trans R Soc Lond B Biol Sci. 2021 Nov 8;376(1837):20200362. doi: 10.1098/rstb.2020.0362. Epub 2021 Sep 20. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 34538146 Free PMC article.
-
Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan.Environ Health Perspect. 2010 Nov;118(11):1507-14. doi: 10.1289/ehp.0901389. Environ Health Perspect. 2010. PMID: 20576580 Free PMC article. Review.
-
Context-dependent effects of large-wildlife declines on small-mammal communities in central Kenya.Ecol Appl. 2015 Mar;25(2):348-60. doi: 10.1890/14-0995.1. Ecol Appl. 2015. PMID: 26263659
Cited by
-
Deterministic processes structure bacterial genetic communities across an urban landscape.Nat Commun. 2019 Jun 14;10(1):2643. doi: 10.1038/s41467-019-10595-1. Nat Commun. 2019. PMID: 31201324 Free PMC article.
-
Disease ecology, health and the environment: a framework to account for ecological and socio-economic drivers in the control of neglected tropical diseases.Philos Trans R Soc Lond B Biol Sci. 2017 Jun 5;372(1722):20160128. doi: 10.1098/rstb.2016.0128. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28438917 Free PMC article.
-
Relationship between Flooding and Out Break of Infectious Diseasesin Kenya: A Review of the Literature.J Environ Public Health. 2018 Oct 17;2018:5452938. doi: 10.1155/2018/5452938. eCollection 2018. J Environ Public Health. 2018. PMID: 30416526 Free PMC article. Review.
-
Advancing sustainable development goals through immunization: a literature review.Global Health. 2021 Aug 26;17(1):95. doi: 10.1186/s12992-021-00745-w. Global Health. 2021. PMID: 34446050 Free PMC article. Review.
-
Ecosystem perspectives are needed to manage zoonotic risks in a changing climate.BMJ. 2020 Nov 13;371:m3389. doi: 10.1136/bmj.m3389. BMJ. 2020. PMID: 33187958 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

