Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control
- PMID: 28438910
- PMCID: PMC5413869
- DOI: 10.1098/rstb.2016.0117
Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control
Abstract
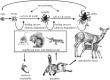
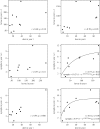
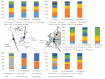
Lyme disease is the most common tick-borne disease in temperate regions of North America, Europe and Asia, and the number of reported cases has increased in many regions as landscapes have been altered. Although there has been extensive work on the ecology and epidemiology of this disease in both Europe and North America, substantial uncertainty exists about fundamental aspects that determine spatial and temporal variation in both disease risk and human incidence, which hamper effective and efficient prevention and control. Here we describe areas of consensus that can be built on, identify areas of uncertainty and outline research needed to fill these gaps to facilitate predictive models of disease risk and the development of novel disease control strategies. Key areas of uncertainty include: (i) the precise influence of deer abundance on tick abundance, (ii) how tick populations are regulated, (iii) assembly of host communities and tick-feeding patterns across different habitats, (iv) reservoir competence of host species, and (v) pathogenicity for humans of different genotypes of Borrelia burgdorferi Filling these knowledge gaps will improve Lyme disease prevention and control and provide general insights into the drivers and dynamics of this emblematic multi-host-vector-borne zoonotic disease.This article is part of the themed issue 'Conservation, biodiversity and infectious disease: scientific evidence and policy implications'.
Keywords: Borrelia burgdorferi; Ixodes; dilution effect; emerging infectious disease; epidemiology.
© 2017 The Author(s).
Conflict of interest statement
We have no competing interests.
Figures


References
-
- CDC. 2016. Lyme disease. See http://www.cdc.gov/lyme/index.html (27 May 2016).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

