Pyrazinoic Acid Inhibits a Bifunctional Enzyme in Mycobacterium tuberculosis
- PMID: 28438933
- PMCID: PMC5487608
- DOI: 10.1128/AAC.00070-17
Pyrazinoic Acid Inhibits a Bifunctional Enzyme in Mycobacterium tuberculosis
Abstract
Pyrazinamide (PZA), an indispensable component of modern tuberculosis treatment, acts as a key sterilizing drug. While the mechanism of activation of this prodrug into pyrazinoic acid (POA) by Mycobacterium tuberculosis has been extensively studied, not all molecular determinants that confer resistance to this mysterious drug have been identified. Here, we report how a new PZA resistance determinant, the Asp67Asn substitution in Rv2783, confers M. tuberculosis resistance to PZA. Expression of the mutant allele but not the wild-type allele in M. tuberculosis recapitulates the PZA resistance observed in clinical isolates. In addition to catalyzing the metabolism of RNA and single-stranded DNA, Rv2783 also metabolized ppGpp, an important signal transducer involved in the stringent response in bacteria. All catalytic activities of the wild-type Rv2783 but not the mutant were significantly inhibited by POA. These results, which indicate that Rv2783 is a target of PZA, provide new insight into the molecular mechanism of the sterilizing activity of this drug and a basis for improving the molecular diagnosis of PZA resistance and developing evolved PZA derivatives to enhance its antituberculosis activity.
Keywords: PNPase; antibiotic resistance; drug target; ppGpp; pyrazinamide; pyrazinoic acid.
Copyright © 2017 American Society for Microbiology.
Figures
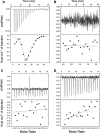
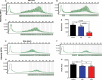
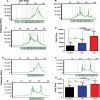


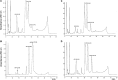
References
-
- World Health Organization. 2016. Global tuberculosis report 2016. World Health Organization, Geneva, Switzerland.
-
- Dawson R, Diacon AH, Everitt D, van Niekerk C, Donald PR, Burger DA, Schall R, Spigelman M, Conradie A, Eisenach K, Venter A, Ive P, Page-Shipp L, Variava E, Reither K, Ntinginya NE, Pym A, von Groote-Bidlingmaier F, Mendel CM.. 2015. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomized trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet 385:1738–1747. doi:10.1016/S0140-6736(14)62002-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

