Host and Bacterial Factors Control Susceptibility of Drosophila melanogaster to Coxiella burnetii Infection
- PMID: 28438980
- PMCID: PMC5478956
- DOI: 10.1128/IAI.00218-17
Host and Bacterial Factors Control Susceptibility of Drosophila melanogaster to Coxiella burnetii Infection
Abstract
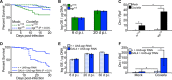
Coxiella burnetii is the causative agent of Q fever, a zoonotic disease that threatens both human and animal health. Due to the paucity of experimental animal models, little is known about how host factors interface with bacterial components and affect pathogenesis. Here, we used Drosophila melanogaster, in conjunction with the biosafety level 2 (BSL2) Nine Mile phase II (NMII) clone 4 strain of C. burnetii, as a model to investigate host and bacterial components implicated in infection. We demonstrate that adult Drosophila flies are susceptible to C. burnetii NMII infection and that this bacterial strain, which activates the immune deficiency (IMD) pathway, is able to replicate and cause mortality in the animals. We show that in the absence of Eiger, the only known tumor necrosis factor (TNF) superfamily homolog in Drosophila, Coxiella-infected flies exhibit reduced mortality from infection. We also demonstrate that the Coxiella type 4 secretion system (T4SS) is critical for the formation of the Coxiella-containing vacuole and establishment of infection in Drosophila Altogether, our data reveal that the Drosophila TNF homolog Eiger and the Coxiella T4SS are implicated in the pathogenesis of C. burnetii in flies. The Drosophila/NMII model mimics relevant aspects of the infection in mammals, such as a critical role of host TNF and the bacterial T4SS in pathogenesis. Our work also demonstrates the usefulness of this BSL2 model to investigate both host and Coxiella components implicated in infection.
Keywords: Eiger; IMD; NMII; Q fever; T4SS; TNF; innate immunity; pathogenesis; tumor necrosis factor.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Coxiella burnetii Inhibits Neutrophil Apoptosis by Exploiting Survival Pathways and Antiapoptotic Protein Mcl-1.Infect Immun. 2018 Mar 22;86(4):e00504-17. doi: 10.1128/IAI.00504-17. Print 2018 Apr. Infect Immun. 2018. PMID: 29311244 Free PMC article.
-
Coxiella burnetii as a useful tool to investigate bacteria-friendly host cell compartments.Int J Med Microbiol. 2018 Jan;308(1):77-83. doi: 10.1016/j.ijmm.2017.09.010. Epub 2017 Sep 14. Int J Med Microbiol. 2018. PMID: 28935173 Review.
-
Biogenesis of the lysosome-derived vacuole containing Coxiella burnetii.Microbes Infect. 2015 Nov-Dec;17(11-12):766-71. doi: 10.1016/j.micinf.2015.08.006. Epub 2015 Aug 29. Microbes Infect. 2015. PMID: 26327296 Free PMC article. Review.
-
Role of Type 4B Secretion System Protein, IcmE, in the Pathogenesis of Coxiella burnetii.Pathogens. 2024 May 14;13(5):405. doi: 10.3390/pathogens13050405. Pathogens. 2024. PMID: 38787259 Free PMC article.
-
Coxiella burnetii Employs the Dot/Icm Type IV Secretion System to Modulate Host NF-κB/RelA Activation.Front Cell Infect Microbiol. 2016 Dec 19;6:188. doi: 10.3389/fcimb.2016.00188. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 28066723 Free PMC article.
Cited by
-
Recent Advances on the Innate Immune Response to Coxiella burnetii.Front Cell Infect Microbiol. 2021 Nov 2;11:754455. doi: 10.3389/fcimb.2021.754455. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34796128 Free PMC article. Review.
-
Coxiella burnetii: international pathogen of mystery.Microbes Infect. 2020 Apr;22(3):100-110. doi: 10.1016/j.micinf.2019.09.001. Epub 2019 Sep 28. Microbes Infect. 2020. PMID: 31574310 Free PMC article. Review.
-
Sexual Dimorphisms in Innate Immunity and Responses to Infection in Drosophila melanogaster.Front Immunol. 2020 Jan 31;10:3075. doi: 10.3389/fimmu.2019.03075. eCollection 2019. Front Immunol. 2020. PMID: 32076419 Free PMC article. Review.
-
Metabolic Plasticity Aids Amphotropism of Coxiella burnetii.Infect Immun. 2021 Nov 16;89(12):e0013521. doi: 10.1128/IAI.00135-21. Epub 2021 Sep 7. Infect Immun. 2021. PMID: 34491791 Free PMC article.
-
Drosophila melanogaster Sting mediates Coxiella burnetii infection by reducing accumulation of reactive oxygen species.Infect Immun. 2024 Mar 12;92(3):e0056022. doi: 10.1128/iai.00560-22. Epub 2024 Feb 16. Infect Immun. 2024. PMID: 38363133 Free PMC article.
References
-
- Schimmer B, Morroy G, Dijkstra F, Schneeberger PM, Weers-Pothoff G, Timen A, Wijkmans C, van der Hoek W. 2008. Large ongoing Q fever outbreak in the south of The Netherlands, 2008. Euro Surveill 13:18939. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

