Acute inflammation reveals GABAA receptor-mediated nociception in mouse dorsal root ganglion neurons via PGE2 receptor 4 signaling
- PMID: 28438981
- PMCID: PMC5408276
- DOI: 10.14814/phy2.13178
Acute inflammation reveals GABAA receptor-mediated nociception in mouse dorsal root ganglion neurons via PGE2 receptor 4 signaling
Abstract
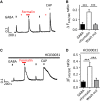
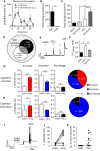
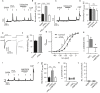
Gamma-aminobutyric acid (GABA) depolarizes dorsal root ganglia (DRG) primary afferent neurons through activation of Cl- permeable GABAA receptors but the physiologic role of GABAA receptors in the peripheral terminals of DRG neurons remains unclear. In this study, we investigated the role of peripheral GABAA receptors in nociception using a mouse model of acute inflammation. In vivo, peripheral administration of the selective GABAA receptor agonist muscimol evoked spontaneous licking behavior, as well as spinal wide dynamic range (WDR) neuron firing, after pre-conditioning with formalin but had no effect in saline-treated mice. GABAA receptor-mediated pain behavior after acute formalin treatment was abolished by the GABAA receptor blocker picrotoxin and cyclooxygenase inhibitor indomethacin. In addition, treatment with prostaglandin E2 (PGE2) was sufficient to reveal muscimol-induced licking behavior. In vitro, GABA induced sub-threshold depolarization in DRG neurons through GABAA receptor activation. Both formalin and PGE2 potentiated GABA-induced Ca2+ transients and membrane depolarization in capsaicin-sensitive nociceptive DRG neurons; these effects were blocked by the prostaglandin E2 receptor 4 (EP4) antagonist AH23848 (10 μmol/L). Furthermore, potentiation of GABA responses by PGE2 was prevented by the selective Nav1.8 antagonist A887826 (100 nmol/L). Although the function of the Na+-K+-2Cl- co-transporter NKCC1 was required to maintain the Cl- ion gradient in isolated DRG neurons, NKCC1 was not required for GABAA receptor-mediated nociceptive behavior after acute inflammation. Taken together, these results demonstrate that GABAA receptors may contribute to the excitation of peripheral sensory neurons in inflammation through a combined effect involving PGE2-EP4 signaling and Na+ channel sensitization.
Keywords: EP4 receptors; Gamma‐aminobutyric acid; TTX‐resistant sodium channels; formalin test; peripheral sensitization; prostaglandin E2.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures
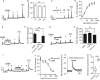


References
-
- Barry, P. H. 1994. JPCalc, a software package for calculating liquid junction potential corrections in patch‐clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods 51:107–116. - PubMed
-
- Berta, T. , Poirot O., Pertin M., Ji R. R., Kellenberger S., and Decosterd I.. 2008. Transcriptional and functional profiles of voltage‐gated Na(+) channels in injured and non‐injured DRG neurons in the SNI model of neuropathic pain. Mol. Cell Neurosci. 37:196–208. - PubMed
-
- Bravo‐Hernandez, M. , Feria‐Morales L. A., Torres‐Lopez J. E., Cervantes‐Duran C., Delgado‐Lezama R., Granados‐Soto V., et al. 2014. Evidence for the participation of peripheral alpha5 subunit‐containing GABAA receptors in GABAA agonists‐induced nociception in rats. Eur. J. Pharmacol. 734:91–97. - PubMed
-
- Carlton, S. M. , Zhou S., and Coggeshall R. E.. 1999. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience 93:713–722. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

