Microglial NFκB-TNFα hyperactivation induces obsessive-compulsive behavior in mouse models of progranulin-deficient frontotemporal dementia
- PMID: 28438992
- PMCID: PMC5441749
- DOI: 10.1073/pnas.1700477114
Microglial NFκB-TNFα hyperactivation induces obsessive-compulsive behavior in mouse models of progranulin-deficient frontotemporal dementia
Abstract
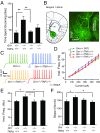
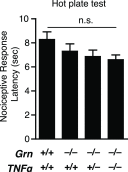
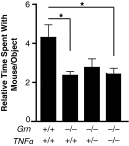
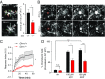
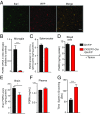
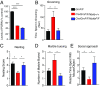
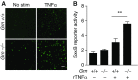
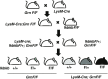
Frontotemporal dementia (FTD) is the second most common dementia before 65 years of age. Haploinsufficiency in the progranulin (GRN) gene accounts for 10% of all cases of familial FTD. GRN mutation carriers have an increased risk of autoimmune disorders, accompanied by elevated levels of tissue necrosis factor (TNF) α. We examined behavioral alterations related to obsessive-compulsive disorder (OCD) and the role of TNFα and related signaling pathways in FTD patients with GRN mutations and in mice lacking progranulin (PGRN). We found that patients and mice with GRN mutations displayed OCD and self-grooming (an OCD-like behavior in mice), respectively. Furthermore, medium spiny neurons in the nucleus accumbens, an area implicated in development of OCD, display hyperexcitability in PGRN knockout mice. Reducing levels of TNFα in PGRN knockout mice abolished excessive self-grooming and the associated hyperexcitability of medium spiny neurons of the nucleus accumbens. In the brain, PGRN is highly expressed in microglia, which are a major source of TNFα. We therefore deleted PGRN specifically in microglia and found that it was sufficient to induce excessive grooming. Importantly, excessive grooming in these mice was prevented by inactivating nuclear factor κB (NF-κB) in microglia/myeloid cells. Our findings suggest that PGRN deficiency leads to excessive NF-κB activation in microglia and elevated TNFα signaling, which in turn lead to hyperexcitability of medium spiny neurons and OCD-like behavior.
Keywords: FTD; OCD; TNF; microglia; progranulin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Progranulin deficiency induces overactivation of WNT5A expression via TNF-α/NF-κB pathway in peripheral cells from frontotemporal dementia-linked granulin mutation carriers.J Psychiatry Neurosci. 2016 Jun;41(4):225-39. doi: 10.1503/jpn.150131. J Psychiatry Neurosci. 2016. PMID: 26624524 Free PMC article.
-
Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury.J Clin Invest. 2012 Nov;122(11):3955-9. doi: 10.1172/JCI63113. Epub 2012 Oct 8. J Clin Invest. 2012. PMID: 23041626 Free PMC article.
-
Network analysis of the progranulin-deficient mouse brain proteome reveals pathogenic mechanisms shared in human frontotemporal dementia caused by GRN mutations.Acta Neuropathol Commun. 2020 Oct 7;8(1):163. doi: 10.1186/s40478-020-01037-x. Acta Neuropathol Commun. 2020. PMID: 33028409 Free PMC article.
-
New insights and therapeutic opportunities for progranulin-deficient frontotemporal dementia.Curr Opin Neurobiol. 2022 Feb;72:131-139. doi: 10.1016/j.conb.2021.10.001. Epub 2021 Nov 23. Curr Opin Neurobiol. 2022. PMID: 34826653 Review.
-
Progranulin as a therapeutic target for dementia.Expert Opin Ther Targets. 2018 Jul;22(7):579-585. doi: 10.1080/14728222.2018.1487951. Epub 2018 Jun 22. Expert Opin Ther Targets. 2018. PMID: 29889573 Review.
Cited by
-
Stable Cells with NF-κB-ZsGreen Fused Genes Created by TALEN Editing and Homology Directed Repair for Screening Anti-inflammation Drugs.J Inflamm Res. 2021 Mar 17;14:917-928. doi: 10.2147/JIR.S298938. eCollection 2021. J Inflamm Res. 2021. PMID: 33762839 Free PMC article.
-
Preclinical Interventions in Mouse Models of Frontotemporal Dementia Due to Progranulin Mutations.Neurotherapeutics. 2023 Jan;20(1):140-153. doi: 10.1007/s13311-023-01348-6. Epub 2023 Feb 13. Neurotherapeutics. 2023. PMID: 36781744 Free PMC article. Review.
-
Differential effects of partial and complete loss of TREM2 on microglial injury response and tauopathy.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):10172-10177. doi: 10.1073/pnas.1811411115. Epub 2018 Sep 19. Proc Natl Acad Sci U S A. 2018. PMID: 30232263 Free PMC article.
-
Loss of TREM2 rescues hyperactivation of microglia, but not lysosomal deficits and neurotoxicity in models of progranulin deficiency.EMBO J. 2022 Feb 15;41(4):e109108. doi: 10.15252/embj.2021109108. Epub 2022 Jan 12. EMBO J. 2022. PMID: 35019161 Free PMC article.
-
Neuroinflammation in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia and the Interest of Induced Pluripotent Stem Cells to Study Immune Cells Interactions With Neurons.Front Mol Neurosci. 2021 Dec 14;14:767041. doi: 10.3389/fnmol.2021.767041. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34970118 Free PMC article. Review.
References
-
- Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci. 2007;1121:528–545. - PubMed
-
- Baker M, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Cruts M, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

