Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis
- PMID: 28438994
- PMCID: PMC5441709
- DOI: 10.1073/pnas.1611776114
Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis
Abstract
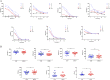
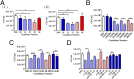
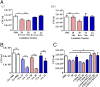
The role of Igs in natural protection against infection by Mycobacterium tuberculosis (Mtb), the causative agent of TB, is controversial. Although passive immunization with mAbs generated against mycobacterial antigens has shown protective efficacy in murine models of infection, studies in B cell-depleted animals only showed modest phenotypes. We do not know if humans make protective antibody responses. Here, we investigated whether healthcare workers in a Beijing TB hospital-who, although exposed to suprainfectious doses of pathogenic Mtb, remain healthy-make antibody responses that are effective in protecting against infection by Mtb. We tested antibodies isolated from 48 healthcare workers and compared these with 12 patients with active TB. We found that antibodies from 7 of 48 healthcare workers but none from active TB patients showed moderate protection against Mtb in an aerosol mouse challenge model. Intriguingly, three of seven healthcare workers who made protective antibody responses had no evidence of prior TB infection by IFN-γ release assay. There was also good correlation between protection observed in vivo and neutralization of Mtb in an in vitro human whole-blood assay. Antibodies mediating protection were directed against the surface of Mtb and depended on both immune complexes and CD4+ T cells for efficacy. Our results indicate that certain individuals make protective antibodies against Mtb and challenge paradigms about the nature of an effective immune response to TB.
Keywords: TB; TB restrictors; antibodies; humoral immunity; immune complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. - PubMed
-
- Kaufmann SH, et al. Progress in tuberculosis vaccine development and host-directed therapies–a state of the art review. Lancet Respir Med. 2014;2:301–320. - PubMed
-
- Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178:7222–7234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

