Impact of Membrane Phospholipid Alterations in Escherichia coli on Cellular Function and Bacterial Stress Adaptation
- PMID: 28439040
- PMCID: PMC5472821
- DOI: 10.1128/JB.00849-16
Impact of Membrane Phospholipid Alterations in Escherichia coli on Cellular Function and Bacterial Stress Adaptation
Abstract
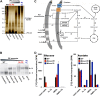
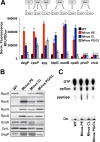
Bacteria have evolved multiple strategies to sense and rapidly adapt to challenging and ever-changing environmental conditions. The ability to alter membrane lipid composition, a key component of the cellular envelope, is crucial for bacterial survival and adaptation in response to environmental stress. However, the precise roles played by membrane phospholipids in bacterial physiology and stress adaptation are not fully elucidated. The goal of this study was to define the role of membrane phospholipids in adaptation to stress and maintenance of bacterial cell fitness. By using genetically modified strains in which the membrane phospholipid composition can be systematically manipulated, we show that alterations in major Escherichia coli phospholipids transform these cells globally. We found that alterations in phospholipids impair the cellular envelope structure and function, the ability to form biofilms, and bacterial fitness and cause phospholipid-dependent susceptibility to environmental stresses. This study provides an unprecedented view of the structural, signaling, and metabolic pathways in which bacterial phospholipids participate, allowing the design of new approaches in the investigation of lipid-dependent processes involved in bacterial physiology and adaptation.IMPORTANCE In order to cope with and adapt to a wide range of environmental conditions, bacteria have to sense and quickly respond to fluctuating conditions. In this study, we investigated the effects of systematic and controlled alterations in bacterial phospholipids on cell shape, physiology, and stress adaptation. We provide new evidence that alterations of specific phospholipids in Escherichia coli have detrimental effects on cellular shape, envelope integrity, and cell physiology that impair biofilm formation, cellular envelope remodeling, and adaptability to environmental stresses. These findings hold promise for future antibacterial therapies that target bacterial lipid biosynthesis.
Keywords: membranes; metabolism; phospholipids; physiology; stress adaptation; stress response.
Copyright © 2017 American Society for Microbiology.
Figures



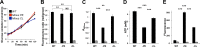


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

