Translation, but not transfection limits clinically relevant, exogenous mRNA based induction of alpha-4 integrin expression on human mesenchymal stem cells
- PMID: 28439079
- PMCID: PMC5430815
- DOI: 10.1038/s41598-017-01304-3
Translation, but not transfection limits clinically relevant, exogenous mRNA based induction of alpha-4 integrin expression on human mesenchymal stem cells
Abstract
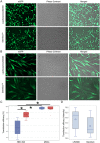
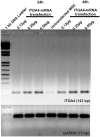
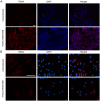
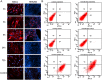
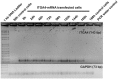
Mesenchymal stem cells (MSCs) represent promising resource of cells for regenerative medicine in neurological disorders. However, efficient and minimally invasive methods of MSCs delivery to the brain still have to be developed. Intra-arterial route is very promising, but MSCs are missing machinery for diapedesis through blood-brain barrier. Thus, here we have tested a mRNA-based method to induce transient expression of ITGA4, an adhesion molecule actively involved in cell extravasation. We observed that transfection with an ITGA4-mRNA construct bearing a conventional cap analogue (7-methylguanosine) failed to produce ITGA4 protein, but exogenous ITGA4-mRNA was detected in transfected MSCs. This indicates that not transfection, but rather translation being the major roadblock. Stabilization of ITGA4-mRNA with SSB proteins resulted in ITGA4 protein synthesis in HEK293 cells only, whereas in MSCs, satisfactory results were obtained only after using an anti-reverse-cap-analogue (ARCA). The presence of ITGA4 protein in MSCs was transient and lasted for up to 24 h after transfection. Membranous location was confirmed by flow cytometry of viable non-permeabilized cells using anti-ITGA4 antibody. The mRNA-based expression of itga4 transgene is potentially sufficient for diapedesis after intra-arterial delivery. To conclude, mRNA-based engineering of stem cells is a rapid and integration-free method and attractive from the perspective of potential future clinical application.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

