ETS Related Gene mediated Androgen Receptor Aggregation and Endoplasmic Reticulum Stress in Prostate Cancer Development
- PMID: 28439080
- PMCID: PMC5430720
- DOI: 10.1038/s41598-017-01187-4
ETS Related Gene mediated Androgen Receptor Aggregation and Endoplasmic Reticulum Stress in Prostate Cancer Development
Erratum in
-
Erratum: ETS Related Gene mediated Androgen Receptor Aggregation and Endoplasmic Reticulum Stress in Prostate Cancer Development.Sci Rep. 2017 Sep 6;7(1):10693. doi: 10.1038/s41598-017-09612-4. Sci Rep. 2017. PMID: 28878398 Free PMC article.
Abstract
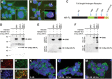
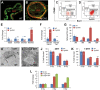
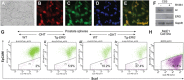
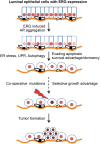
Mechanistic studies of deregulated ERG in prostate cancer and other cancers continue to enhance its role in cancer biology and its utility as a biomarker and therapeutic target. Here, we show that ERG, through its physical interaction with androgen receptor, induces AR aggregation and endoplasmic reticulum stress in the prostate glands of ERG transgenic mice. Histomorphological alterations and the expression of ER stress sensors Atf6, Ire1α, Perk, their downstream effectors Grp78/BiP and eIF2α in ERG transgenic mouse prostate glands indicate the presence of chronic ER stress. Transient activation of apoptotic cell death during early age correlated well with the differential regulation of ER stress sensors, in particular Perk. Epithelial cells derived from ERG transgenic mouse prostates have increased prostasphere formation with resistance to radiation induced cell death. Continued activation of cell survival factors, Atf6 and Ire1α during chronic ER stress due to presence of ERG in prostate epithelium induces survival pathways and provides a selection pressure in the continuum of ERG dependent neoplastic process. These novel insights will enhance the understanding of the mechanistic functions of ERG in prostate tumor biology and towards development of early targeted therapeutic strategies for prostate cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
ERG signaling in prostate cancer is driven through PRMT5-dependent methylation of the Androgen Receptor.Elife. 2016 May 16;5:e13964. doi: 10.7554/eLife.13964. Elife. 2016. PMID: 27183006 Free PMC article.
-
ERG-Mediated Coregulator Complex Formation Maintains Androgen Receptor Signaling in Prostate Cancer.Cancer Res. 2020 Nov 1;80(21):4612-4619. doi: 10.1158/0008-5472.CAN-20-2044. Epub 2020 Sep 15. Cancer Res. 2020. PMID: 32934023
-
TMPRSS2:ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer.Cancer Res. 2006 Nov 15;66(22):10658-63. doi: 10.1158/0008-5472.CAN-06-1871. Cancer Res. 2006. PMID: 17108102
-
Androgen receptor and prostate cancer.Prostate Cancer Prostatic Dis. 2007;10(2):114-8. doi: 10.1038/sj.pcan.4500936. Epub 2007 Feb 13. Prostate Cancer Prostatic Dis. 2007. PMID: 17297502 Review.
-
The oncogene ERG: a key factor in prostate cancer.Oncogene. 2016 Jan 28;35(4):403-14. doi: 10.1038/onc.2015.109. Epub 2015 Apr 27. Oncogene. 2016. PMID: 25915839 Review.
Cited by
-
Oxidative stress as a catalyst in prostate cancer progression: unraveling molecular mechanisms and exploring therapeutic interventions.Discov Oncol. 2025 Apr 3;16(1):457. doi: 10.1007/s12672-025-02245-4. Discov Oncol. 2025. PMID: 40178629 Free PMC article. Review.
-
Riluzole induces AR degradation via endoplasmic reticulum stress pathway in androgen-dependent and castration-resistant prostate cancer cells.Prostate. 2019 Feb;79(2):140-150. doi: 10.1002/pros.23719. Epub 2018 Oct 2. Prostate. 2019. PMID: 30280407 Free PMC article.
-
The non-canonical mechanism of ER stress-mediated progression of prostate cancer.J Exp Clin Cancer Res. 2021 Sep 14;40(1):289. doi: 10.1186/s13046-021-02066-7. J Exp Clin Cancer Res. 2021. PMID: 34521429 Free PMC article.
-
Systematic analysis reveals molecular characteristics of ERG-negative prostate cancer.Sci Rep. 2018 Aug 27;8(1):12868. doi: 10.1038/s41598-018-30325-9. Sci Rep. 2018. PMID: 30150711 Free PMC article.
-
Recent advances in prostate cancer research: large-scale genomic analyses reveal novel driver mutations and DNA repair defects.F1000Res. 2018 Aug 2;7:F1000 Faculty Rev-1173. doi: 10.12688/f1000research.14499.1. eCollection 2018. F1000Res. 2018. PMID: 30135717 Free PMC article. Review.
References
-
- Wilt TJ. The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy with watchful waiting for men with clinically localized prostate cancer. J Natl Cancer Inst Monogr. 2012;2012:184–90. doi: 10.1093/jncimonographs/lgs041. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

