Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects
- PMID: 28439098
- PMCID: PMC5822462
- DOI: 10.1038/mp.2017.85
Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects
Abstract
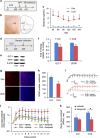
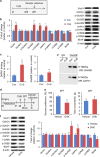
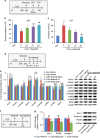
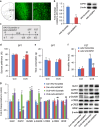
Several preclinical studies have reported the rapid antidepressant effects of N-methyl-D-aspartate receptor (NMDAR) antagonists, although the underlying mechanisms are still unclear. Death-associated protein kinase 1 (DAPK1) couples GluN2B subunits at extrasynaptic sites to regulate NMDAR channel conductance. In the present study, we found that chronic unpredictable stress (CUS) induced extracellular glutamate accumulation, accompanied by an increase in the DAPK1-NMDAR interaction, the high expression of DAPK1 and phosphorylated GluN2B at Ser1303, a decrease in phosphorylated DAPK1 at Ser308 and synaptic protein deficits in the rat medial prefrontal cortex (mPFC). CUS also enhanced GluN2B-mediated NMDA currents and extrasynaptic responses that were induced by bursts of high-frequency stimulation, which may be associated with the loss of astrocytes and low expression of glutamate transporter-1 (GLT-1). The blockade of GLT-1 in the mPFC was sufficient to induce depressive-like behavior and cause similar molecular changes. Selective GluN2B antagonist, DAPK1 knockdown by adeno-associated virus-mediated short-hairpin RNA or a pharmacological inhibitor, and the uncoupling of DAPK1 from the NMDAR GluN2B subunit produced rapid antidepressant-like effects and reversed CUS-induced alterations in the mPFC. The inhibition of DAPK1 and its interaction with GluN2B subunit in the mPFC also rescued CUS-induced depressive-like behavior 7 days after treatment. A selective GluN2B antagonist did not have rewarding effects in the conditioned place preference paradigm. Altogether, our findings suggest that the DAPK1 interaction with the NMDAR GluN2B subunit acts as a critical component in the pathophysiology of depression and is a potential target for new antidepressant treatments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism.Behav Brain Res. 2015;287:89-95. doi: 10.1016/j.bbr.2015.03.023. Epub 2015 Mar 21. Behav Brain Res. 2015. PMID: 25800971 Free PMC article.
-
Rapastinel (GLYX-13) has therapeutic potential for the treatment of post-traumatic stress disorder: Characterization of a NMDA receptor-mediated metaplasticity process in the medial prefrontal cortex of rats.Behav Brain Res. 2015 Nov 1;294:177-85. doi: 10.1016/j.bbr.2015.07.039. Epub 2015 Jul 22. Behav Brain Res. 2015. PMID: 26210936 Free PMC article.
-
Ifenprodil rapidly ameliorates depressive-like behaviors, activates mTOR signaling and modulates proinflammatory cytokines in the hippocampus of CUMS rats.Psychopharmacology (Berl). 2020 May;237(5):1421-1433. doi: 10.1007/s00213-020-05469-0. Epub 2020 Mar 4. Psychopharmacology (Berl). 2020. PMID: 32130432
-
Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition.Neuropharmacology. 2016 Jan;100:17-26. doi: 10.1016/j.neuropharm.2015.07.028. Epub 2015 Jul 26. Neuropharmacology. 2016. PMID: 26211972 Review.
-
Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects.Brain Res Bull. 2013 Apr;93:32-8. doi: 10.1016/j.brainresbull.2012.10.005. Epub 2012 Oct 23. Brain Res Bull. 2013. PMID: 23089362 Review.
Cited by
-
AKT and MAPK signaling pathways in hippocampus reveals the pathogenesis of depression in four stress-induced models.Transl Psychiatry. 2023 Jun 12;13(1):200. doi: 10.1038/s41398-023-02486-3. Transl Psychiatry. 2023. PMID: 37308476 Free PMC article.
-
Targeting cis-p-tau and neuro-related gene expression in traumatic brain injury: therapeutic insights from TC-DAPK6 treatment in mice.Mol Biol Rep. 2024 Sep 25;51(1):1010. doi: 10.1007/s11033-024-09945-0. Mol Biol Rep. 2024. PMID: 39320385
-
Death-associated protein kinase 1 as a therapeutic target for Alzheimer's disease.Transl Neurodegener. 2024 Jan 9;13(1):4. doi: 10.1186/s40035-023-00395-5. Transl Neurodegener. 2024. PMID: 38195518 Free PMC article. Review.
-
Photobiomodulation Therapy Ameliorates Glutamatergic Dysfunction in Mice with Chronic Unpredictable Mild Stress-Induced Depression.Oxid Med Cell Longev. 2021 Mar 29;2021:6678276. doi: 10.1155/2021/6678276. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33859781 Free PMC article.
-
Reduction of BDNF Levels and Biphasic Changes in Glutamate Release in the Prefrontal Cortex Correlate with Susceptibility to Chronic Stress-Induced Anhedonia.eNeuro. 2023 Nov 21;10(11):ENEURO.0406-23.2023. doi: 10.1523/ENEURO.0406-23.2023. Print 2023 Nov. eNeuro. 2023. PMID: 37989582 Free PMC article.
References
-
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370: 851–858. - PubMed
-
- Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry 2000; 47: 586–593. - PubMed
-
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 2007; 62: 1310–1316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

