Clinical Feasibility of Monitoring Enoxaparin Anti-Xa Concentrations: Are We Getting It Right?
- PMID: 28439136
- PMCID: PMC5396989
- DOI: 10.1310/hpj5203-214
Clinical Feasibility of Monitoring Enoxaparin Anti-Xa Concentrations: Are We Getting It Right?
Abstract
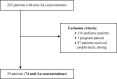
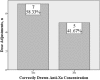
Background: Anti-Xa monitoring is utilized to measure the extent of anticoagulation in certain patient populations receiving enoxaparin. It is essential to accurately obtain this pharmacodynamic marker for safe and effective anticoagulation management. Objectives: To determine the frequency of correctly drawn anti-Xa concentrations in accordance with predefined institutional criteria and to determine the number of dose adjustments implemented based on incorrectly drawn anti-Xa concentrations. Methods: This was a retrospective, single-center, cohort study among adult patients who received treatment doses of enoxaparin with measured anti-Xa concentrations. Patients were excluded if they were pregnant, on hemodialysis, or received prophylactic dosing. Anti-Xa levels were defined as correctly measured if they were drawn 3 to 5 hours after the dose during steady state concentrations. Descriptive statistics were performed and analyzed via SPSS software. Results: Overall, 203 patients were reviewed and 59 patients with 74 anti-Xa levels were included. The majority of anti-Xa levels (57/74; 77%) were drawn incorrectly and often resulted in collection of repeat anti-Xa samples. There were 12 documented dose adjustments and approximately 42% (5/12) were based on incorrectly drawn anti-Xa levels. Anti-Xa levels were within target range approximately 45% of the time. Conclusions: Enoxaparin anti-Xa concentrations are frequently drawn incorrectly and dose adjustments are often performed based on these unsupported anti-Xa levels. This may present a potential risk to compromise patient safety.
Keywords: anti-Xa monitoring; enoxaparin; low molecular weight heparin; pharmacodynamic monitoring.
Figures

References
-
- Aguilar D, Goldhaber SZ.. Clinical uses of low-molecular-weight heparins. Chest. 1999; 115( 5): 1418– 1423. - PubMed
-
- Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997; 337( 10): 688– 698. - PubMed
-
- Hirsh J, Levine MN.. Low molecular weight heparin. Blood. 1992; 79( 1): 1– 17. - PubMed
-
- Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, Dalen JE.. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1998; 114 ( 5 suppl): 489S– 510S. - PubMed
-
- Frydman A. Low-molecular-weight heparins: An overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996; 26( suppl 2): 24– 38. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

