Alveolar Fluid Clearance in Pathologically Relevant Conditions: In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome
- PMID: 28439268
- PMCID: PMC5383664
- DOI: 10.3389/fimmu.2017.00371
Alveolar Fluid Clearance in Pathologically Relevant Conditions: In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome
Abstract
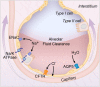
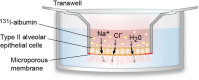
Critically ill patients with respiratory failure from acute respiratory distress syndrome (ARDS) have reduced ability to clear alveolar edema fluid. This reduction in alveolar fluid clearance (AFC) contributes to the morbidity and mortality in ARDS. Thus, it is important to understand why AFC is reduced in ARDS in order to design targeted therapies. In this review, we highlight experiments that have advanced our understanding of ARDS pathogenesis, with particular reference to the alveolar epithelium. First, we review how vectorial ion transport drives the clearance of alveolar edema fluid in the uninjured lung. Next, we describe how alveolar edema fluid is less effectively cleared in lungs affected by ARDS and describe selected in vitro and in vivo experiments that have elucidated some of the molecular mechanisms responsible for the reduced AFC. Finally, we describe one potential therapy that targets this pathway: bone marrow-derived mesenchymal stem (stromal) cells (MSCs). Based on preclinical studies, MSCs enhance AFC and promote the resolution of pulmonary edema and thus may offer a promising cell-based therapy for ARDS.
Keywords: acute respiratory distress syndrome; alveolar fluid clearance; mesenchymal stem (stromal) cells; pulmonary edema; vectorial ion transport.
Figures
Similar articles
-
Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome.Am J Respir Crit Care Med. 2015 Jul 15;192(2):191-9. doi: 10.1164/rccm.201501-0020OC. Am J Respir Crit Care Med. 2015. PMID: 25932660
-
Specialized Pro-resolving Mediators Regulate Alveolar Fluid Clearance during Acute Respiratory Distress Syndrome.Chin Med J (Engl). 2018 Apr 20;131(8):982-989. doi: 10.4103/0366-6999.229890. Chin Med J (Engl). 2018. PMID: 29664060 Free PMC article. Review.
-
Mechanisms of impaired alveolar fluid clearance.Anat Rec (Hoboken). 2025 Apr;308(4):1026-1039. doi: 10.1002/ar.25166. Epub 2023 Jan 23. Anat Rec (Hoboken). 2025. PMID: 36688689 Free PMC article. Review.
-
In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome.Stem Cells Transl Med. 2015 Oct;4(10):1199-213. doi: 10.5966/sctm.2015-0021. Epub 2015 Aug 18. Stem Cells Transl Med. 2015. PMID: 26285659 Free PMC article.
-
Gas Exchange Disturbances Regulate Alveolar Fluid Clearance during Acute Lung Injury.Front Immunol. 2017 Jul 4;8:757. doi: 10.3389/fimmu.2017.00757. eCollection 2017. Front Immunol. 2017. PMID: 28725223 Free PMC article. Review.
Cited by
-
Lipoxin A4 ameliorates alveolar fluid clearance disturbance in lipopolysaccharide-induced lung injury via aquaporin 5 and MAPK signaling pathway.J Thorac Dis. 2019 Aug;11(8):3599-3608. doi: 10.21037/jtd.2019.08.86. J Thorac Dis. 2019. PMID: 31559067 Free PMC article.
-
Cellular Mechanisms of Lung Injury: Current Perspectives.Clin Chest Med. 2024 Dec;45(4):821-833. doi: 10.1016/j.ccm.2024.08.004. Epub 2024 Sep 20. Clin Chest Med. 2024. PMID: 39443000 Review.
-
Restricted, optimized or liberal fluid strategy in thoracic surgery: A narrative review.Saudi J Anaesth. 2021 Jul-Sep;15(3):324-334. doi: 10.4103/sja.sja_1155_20. Epub 2021 Jun 19. Saudi J Anaesth. 2021. PMID: 34764839 Free PMC article. Review.
-
Development of a physiomimetic model of acute respiratory distress syndrome by using ECM hydrogels and organ-on-a-chip devices.Front Pharmacol. 2022 Sep 2;13:945134. doi: 10.3389/fphar.2022.945134. eCollection 2022. Front Pharmacol. 2022. PMID: 36188621 Free PMC article.
-
Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury.J Infect Dis. 2019 Jan 7;219(2):186-196. doi: 10.1093/infdis/jiy478. J Infect Dis. 2019. PMID: 30085072 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

