Engineering Muscle Networks in 3D Gelatin Methacryloyl Hydrogels: Influence of Mechanical Stiffness and Geometrical Confinement
- PMID: 28439516
- PMCID: PMC5383707
- DOI: 10.3389/fbioe.2017.00022
Engineering Muscle Networks in 3D Gelatin Methacryloyl Hydrogels: Influence of Mechanical Stiffness and Geometrical Confinement
Abstract
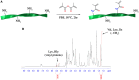
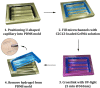
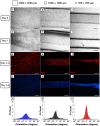
In this work, the influence of mechanical stiffness and geometrical confinement on the 3D culture of myoblast-laden gelatin methacryloyl (GelMA) photo-crosslinkable hydrogels was evaluated in terms of in vitro myogenesis. We formulated a set of cell-laden GelMA hydrogels with a compressive modulus in the range 1 ÷ 17 kPa, obtained by varying GelMA concentration and degree of cross-linking. C2C12 myoblasts were chosen as the cell model to investigate the supportiveness of different GelMA hydrogels toward myotube formation up to 2 weeks. Results showed that the hydrogels with a stiffness in the range 1 ÷ 3 kPa provided enhanced support to C2C12 differentiation in terms of myotube number, rate of formation, and space distribution. Finally, we studied the influence of geometrical confinement on myotube orientation by confining cells within thin hydrogel slabs having different cross sections: (i) 2,000 μm × 2,000 μm, (ii) 1,000 μm × 1,000 μm, and (iii) 500 μm × 500 μm. The obtained results showed that by reducing the cross section, i.e., by increasing the level of confinement-myotubes were more closely packed and formed aligned myostructures that better mimicked the native morphology of skeletal muscle.
Keywords: C2C12 differentiation; gelatin methacryloyl; geometrical confinement; hydrogel stiffness; skeletal muscle.
Figures


Similar articles
-
Enhanced Electroactivity, Mechanical Properties, and Printability through the Addition of Graphene Oxide to Photo-Cross-linkable Gelatin Methacryloyl Hydrogel.ACS Biomater Sci Eng. 2021 Jun 14;7(6):2279-2295. doi: 10.1021/acsbiomaterials.0c01734. Epub 2021 May 6. ACS Biomater Sci Eng. 2021. PMID: 33956434
-
Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting.ACS Appl Mater Interfaces. 2018 Aug 15;10(32):26859-26869. doi: 10.1021/acsami.8b06607. Epub 2018 Aug 1. ACS Appl Mater Interfaces. 2018. PMID: 30024722
-
Enhanced skeletal muscle formation on microfluidic spun gelatin methacryloyl (GelMA) fibres using surface patterning and agrin treatment.J Tissue Eng Regen Med. 2018 Nov;12(11):2151-2163. doi: 10.1002/term.2738. Epub 2018 Sep 30. J Tissue Eng Regen Med. 2018. PMID: 30048044
-
Characterizing the Effects of Synergistic Thermal and Photo-Cross-Linking during Biofabrication on the Structural and Functional Properties of Gelatin Methacryloyl (GelMA) Hydrogels.ACS Biomater Sci Eng. 2021 Nov 8;7(11):5175-5188. doi: 10.1021/acsbiomaterials.1c00635. Epub 2021 Oct 1. ACS Biomater Sci Eng. 2021. PMID: 34597013
-
Engineering Microvascular Networks in LED Light-Cured Cell-Laden Hydrogels.ACS Biomater Sci Eng. 2018 Jul 9;4(7):2563-2570. doi: 10.1021/acsbiomaterials.8b00502. Epub 2018 Jun 26. ACS Biomater Sci Eng. 2018. PMID: 33435119
Cited by
-
Engineering Anisotropic Muscle Tissue using Acoustic Cell Patterning.Adv Mater. 2018 Oct;30(43):e1802649. doi: 10.1002/adma.201802649. Epub 2018 Sep 12. Adv Mater. 2018. PMID: 30277617 Free PMC article.
-
In Vivo Printing of Nanoenabled Scaffolds for the Treatment of Skeletal Muscle Injuries.Adv Healthc Mater. 2021 May;10(10):e2002152. doi: 10.1002/adhm.202002152. Epub 2021 Feb 28. Adv Healthc Mater. 2021. PMID: 33644996 Free PMC article.
-
3D Bioprinting of Macroporous Materials Based on Entangled Hydrogel Microstrands.Adv Sci (Weinh). 2020 Jul 19;7(18):2001419. doi: 10.1002/advs.202001419. eCollection 2020 Sep. Adv Sci (Weinh). 2020. PMID: 32999847 Free PMC article.
-
Development of High-Cell-Density Tissue Method for Compressed Modular Bioactuator.Micromachines (Basel). 2022 Oct 12;13(10):1725. doi: 10.3390/mi13101725. Micromachines (Basel). 2022. PMID: 36296079 Free PMC article.
-
Matching mechanical heterogeneity of the native spinal cord augments axon infiltration in 3D-printed scaffolds.Biomaterials. 2023 Apr;295:122061. doi: 10.1016/j.biomaterials.2023.122061. Epub 2023 Feb 16. Biomaterials. 2023. PMID: 36842339 Free PMC article.
References
-
- Buckingham M., Montarras D. (2008). Skeletal Muscle Repair and Regeneration. Netherlands: Springer, 19–44.
LinkOut - more resources
Full Text Sources
Other Literature Sources

