Antipsychotic Prescribing to Patients Diagnosed with Dementia Without a Diagnosis of Psychosis in the Context of National Guidance and Drug Safety Warnings: Longitudinal Study in UK General Practice
- PMID: 28439716
- PMCID: PMC5519656
- DOI: 10.1007/s40264-017-0538-x
Antipsychotic Prescribing to Patients Diagnosed with Dementia Without a Diagnosis of Psychosis in the Context of National Guidance and Drug Safety Warnings: Longitudinal Study in UK General Practice
Erratum in
-
Correction to: Antipsychotic Prescribing to Patients Diagnosed with Dementia Without a Diagnosis of Psychosis in the Context of National Guidance and Drug Safety Warnings: Longitudinal Study in UK General Practice.Drug Saf. 2019 May;42(5):697. doi: 10.1007/s40264-019-00817-2. Drug Saf. 2019. PMID: 30963449 Free PMC article.
Abstract
Introduction: Policy interventions to address inappropriate prescribing of antipsychotic drugs to older people diagnosed with dementia are commonplace. In the UK, warnings were issued by the Medicines Healthcare products Regulatory Agency in 2004, 2009 and 2012 and the National Institute for Health and Care Excellence guidance was published in 2006. It is important to evaluate the impact of such interventions.
Methods: We analysed routinely collected primary-care data from 111,346 patients attending one of 689 general practices contributing to the Clinical Practice Research Datalink to describe the temporal changes in the prescribing of antipsychotic drugs to patients aged 65 years or over diagnosed with dementia without a concomitant psychosis diagnosis from 2001 to 2014 using an interrupted time series and a before-and-after design. Logistic regression methods were used to quantify the impact of patient and practice level variables on prescribing prevalence.
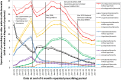
Results: Prescribing of first-generation antipsychotic drugs reduced from 8.9% in 2001 to 1.4% in 2014 (prevalence ratio 2014/2001 adjusted for age, sex and clustering within practices (0.14, 95% confidence interval 0.12-0.16), whereas there was little change for second-generation antipsychotic drugs (1.01, confidence interval 0.94-1.17). Between 2004 and 2012, several policy interventions coincided with a pattern of ups and downs, whereas the 2006 National Institute for Health and Care Excellence guidance was followed by a gradual longer term reduction. Since 2013, the decreasing trend in second-generation antipsychotic drug prescribing has plateaued largely driven by the increasing prescribing of risperidone.
Conclusions: Increased surveillance and evaluation of drug safety warnings and guidance are needed to improve the impact of future interventions.
Conflict of interest statement
Conflict of interest
S Jill Stocks, Evangelos Kontopantelis, Roger T. Webb, Anthony J. Avery, Alistair Burns and Darren M. Ashcroft have no conflicts of interest that are directly relevant to the content of this study.
Ethics approval
The study was approved by the Independent Scientific Advisory Committee for Clinical Practice Research Datalink research (Reference No. 16_054). No further ethics approval was required for the analysis of the data.
Figures

Comment in
-
Good Intentions, But What About Unintended Consequences?Drug Saf. 2017 Aug;40(8):647-649. doi: 10.1007/s40264-017-0554-x. Drug Saf. 2017. PMID: 28660499 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

