A pilot study of patient-centered outcome assessment using PROMIS for patients undergoing colorectal surgery
- PMID: 28439726
- PMCID: PMC5577058
- DOI: 10.1007/s00520-017-3718-4
A pilot study of patient-centered outcome assessment using PROMIS for patients undergoing colorectal surgery
Abstract
Purpose: Few studies have assessed patient-reported outcomes following colorectal surgery. The absence of this information makes it difficult to inform patients about the near-term effects of surgery, beyond outcomes assessed by traditional clinical measures. This study was designed to provide information about the effects of colorectal surgery on physical, mental, and social well-being outcomes.
Methods: The NIH Patient-Reported Outcomes Measurement Information System (PROMIS®) Assessment Center was used to collect patient responses prior to surgery and at their routine postoperative visit. Four domains were selected based on patient consultation and clinical experience: depression, pain interference, ability to participate in social roles and activities, and interest in sexual activity. Multilevel random coefficient models were used to assess the change in scores during the follow-up period and to assess the statistical significance of differences in trends over time associated with key clinical measures.
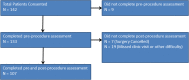
Results: In total, 142 patients were consented, with 107 patients completing pre- and postoperative assessments (75%). Preoperative assessments were typically completed 1 month prior to surgery (mean 29.5 days before, SD = 19.7) and postoperative assessments 1 month after surgery (mean 30.7 days after, SD = 9.2), with a mean of 60.3 days between assessment dates. Patients demonstrated no statistically significant changes in scores for pain interference (-0.18 points, p = 0.80) or the ability to participate in social roles and activities (0.44 points, p = 0.55), but had significant decreases in depression scores between pre- and postoperative assessments (-1.6 points, p = 0.03) and near significant increases in scores for interest in sex (1.5 points, p = 0.06). Pain interference scores for patients with neoadjuvant chemotherapy significantly increased (3.5 points, p = 0.03). Scores for the interest in sex domain decreased (worsened) for patients with oncologic etiology (-3.7 points, p = 0.03). No other differences in score trends by patient characteristics were large enough to be statistically significant at the p < 0.05 threshold.
Conclusion: These data suggest that the majority of patients quickly return to baseline physical, mental, and social function following colorectal surgery. This information can be used preoperatively to counsel patients about the typical impact of colorectal surgery on quality of life.
Keywords: Colorectal surgery; Enhanced recovery after surgery; PROMIS; Patient reported outcomes.
Conflict of interest statement
The authors declare that there are no conflicts of interest to report.
Dr. Stukenborg retains full control of all primary data used in this analysis and the authors agree to allow the journal to review the data if requested. The authors have no possible conflicts of interest in the manuscript, including no financial, consultant, institutional, and other relationships that might lead to bias.
Figures



References
-
- Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, Sloan J, Wenzel K, Chauhan C, Eppard W, Frank ES, Lipscomb J, Raymond SA, Spencer M, Tunis S. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–4255. doi: 10.1200/JCO.2012.42.5967. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- U01 AR052170/AR/NIAMS NIH HHS/United States
- U01 AR057929/AR/NIAMS NIH HHS/United States
- U01 AR052181/AR/NIAMS NIH HHS/United States
- U01 AR057954/AR/NIAMS NIH HHS/United States
- U01 AR057940/AR/NIAMS NIH HHS/United States
- U01 AR052177/AR/NIAMS NIH HHS/United States
- U01 AR057948/AR/NIAMS NIH HHS/United States
- U01 AR057956/AR/NIAMS NIH HHS/United States
- U01 AR052158/AR/NIAMS NIH HHS/United States
- U01 AR057971/AR/NIAMS NIH HHS/United States
- U01 AR057967/AR/NIAMS NIH HHS/United States
- U01 AR052155/AR/NIAMS NIH HHS/United States
- U54 AR057943/AR/NIAMS NIH HHS/United States
- U01 AR052171/AR/NIAMS NIH HHS/United States
- U01 AR057936/AR/NIAMS NIH HHS/United States
- U01 AR052186/AR/NIAMS NIH HHS/United States
- U54 AR057951/AR/NIAMS NIH HHS/United States
- U54 AR057926/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

