Developing the Totality of Evidence for Biosimilars: Regulatory Considerations and Building Confidence for the Healthcare Community
- PMID: 28439817
- PMCID: PMC5443883
- DOI: 10.1007/s40259-017-0218-5
Developing the Totality of Evidence for Biosimilars: Regulatory Considerations and Building Confidence for the Healthcare Community
Abstract
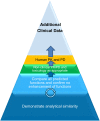
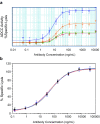
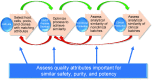
Biosimilars are highly similar versions of approved branded biologics. Unlike generics, they are not exact replicas of reference products. Minor differences between biosimilars and reference products in some aspects are expected; likewise, biosimilar products will differ from each other. The objective of this review is to discuss the challenges associated with the development and approval of biosimilar products that are unique because of their complex structure and specialized manufacturing processes, which can impact not only efficacy but also immunogenicity and safety. Regulatory guidelines recommend a totality-of-evidence approach focused on stepwise development that involves demonstration of structural similarity and functional equivalence. Structural and functional characteristics of the proposed biosimilar are compared with the reference product; similarity of these functions forms the foundation of the biosimilar development program, including potential animal studies, a human pharmacokinetics/pharmacodynamics equivalence study, and a clinical study to confirm similar efficacy, safety, and immunogenicity. The clinical study should be performed in a sensitive population using appropriate endpoints to allow detection of any clinically meaningful differences between the biosimilar and the reference product if such differences exist. In conclusion, development of biosimilars is focused on the minimization of potential differences between the proposed biosimilar and reference product and the establishment of a robust manufacturing process to consistently produce a high-quality biosimilar product.
Conflict of interest statement
Conflicts of Interest
Richard Markus, Jennifer Liu, Monica Ramchandani, Diana Landa, Teresa Born, and Primal Kaur are employees of Amgen Inc. and own Amgen stock.
Funding
Funded by Amgen Inc.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Figures


References
-
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. London, UK. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 30 March 2016.
-
- European Medicines Agency. Guideline on similar biological medicinal products. London, UK. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 30 March 2016.
-
- US Food and Drug Administration. Guidance for industry: biosimilars: questions and answers regarding implementation of the Biologics Price Competition and Innovation Act of 2009. Rockville, MD. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Accessed 30 March 2016.
-
- US Food and Drug Administration. Guidance for industry: quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. Rockville, MD. 2015. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... Accessed 30 March 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

