Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial)
- PMID: 28439953
- PMCID: PMC5523858
- DOI: 10.1002/ajh.24770
Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial)
Erratum in
-
Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial).Am J Hematol. 2018 Mar;93(3):E83. doi: 10.1002/ajh.25012. Epub 2018 Feb 2. Am J Hematol. 2018. PMID: 29411418 No abstract available.
Abstract
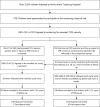
The vast majority of children with sickle cell anemia (SCA) live in Africa, where evidence-based guidelines for primary stroke prevention are lacking. In Kano, Nigeria, we conducted a feasibility trial to determine the acceptability of hydroxyurea therapy for primary stroke prevention in children with abnormal transcranial Doppler (TCD) measurements. Children with SCA and abnormal non-imaging TCD measurements (≥200 cm/s) received moderate fixed-dose hydroxyurea therapy (∼20 mg/kg/day). A comparison group of children with TCD measurements <200 cm/s was followed prospectively. Approximately 88% (330 of 375) of families agreed to be screened, while 87% (29 of 33) of those with abnormal TCD measurements, enrolled in the trial. No participant elected to withdraw from the trial. The average mean corpuscular volume increased from 85.7 fl at baseline to 95.5 fl at 24 months (not all of the children who crossed over had a 24 month visit), demonstrating adherence to hydroxyurea. The comparison group consisted of initially 210 children, of which four developed abnormal TCD measurements, and were started on hydroxyurea. None of the monthly research visits were missed (n = total 603 visits). Two and 10 deaths occurred in the treatment and comparison groups, with mortality rates of 2.69 and 1.81 per 100 patient-years, respectively (P = .67). Our results provide strong evidence, for high family recruitment, retention, and adherence rates, to undertake the first randomized controlled trial with hydroxyurea therapy for primary stroke prevention in children with SCA living in Africa.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
No conflicts of interest to declare.
Figures
References
-
- Adams RJ. Lessons from the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study. J Child Neurol. 2000;15:344–349. - PubMed
-
- Therrell BL, Jr, Lloyd-Puryear MA, Eckman JR, et al. Newborn screening for sickle cell diseases in the United States: A review of data spanning 2 decades. Semin Perinatol. 2015;39:238–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical