Production, characterization, and assessment of a stable analog of the response regulator CheY-phosphate from Thermotoga maritima
- PMID: 28440031
- PMCID: PMC5521581
- DOI: 10.1002/pro.3180
Production, characterization, and assessment of a stable analog of the response regulator CheY-phosphate from Thermotoga maritima
Abstract
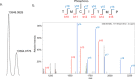
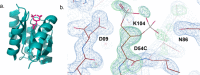
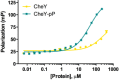
Phosphorylation of CheY promotes association with the flagellar motor and ultimately controls the directional bias of the motor. However, biochemical studies of activated CheY-phosphate have been challenging due to the rapid hydrolysis of the aspartyl-phosphate in vitro. An inert analog of Tm CheY-phosphate, phosphono-CheY, was synthesized by chemical modification and purified by cation-exchange chromatography. Changes in HPLC retention times, chemical assays for phosphate and free thiol, and mass spectrometry experiments demonstrate modification of Cys54 with a phosphonomethyl group. Additionally, a crystal structure showed electron density for the phosphonomethyl group at Cys54, consistent with a modification at that position. Subsequent biochemical experiments confirmed that protein crystals were phosphono-CheY. Isothermal titration calorimetry and fluorescence polarization binding assays demonstrated that phosphono-CheY bound a peptide derived from FliM, a native partner of CheY-phosphate, with a dissociation constant of ∼29 µM, at least sixfold more tightly than unmodified CheY. Taken together these results suggest that Tm phosphono-CheY is a useful and unique analog of Tm CheY-phosphate.
Keywords: chemotaxis; cysteine modification; phosphate-analog; two-component system.
© 2017 The Protein Society.
Figures

Similar articles
-
Synthesis of a Stable Analog of the Phosphorylated Form of CheY: Phosphono-CheY.Methods Mol Biol. 2018;1729:337-343. doi: 10.1007/978-1-4939-7577-8_26. Methods Mol Biol. 2018. PMID: 29429102
-
The structures of T87I phosphono-CheY and T87I/Y106W phosphono-CheY help to explain their binding affinities to the FliM and CheZ peptides.Arch Biochem Biophys. 2008 Nov 15;479(2):105-13. doi: 10.1016/j.abb.2008.08.019. Epub 2008 Sep 5. Arch Biochem Biophys. 2008. PMID: 18801331 Free PMC article.
-
The crystal structure of an activated Thermotoga maritima CheY with N-terminal region of FliM.Int J Biol Macromol. 2013 Mar;54:76-83. doi: 10.1016/j.ijbiomac.2012.12.003. Epub 2012 Dec 10. Int J Biol Macromol. 2013. PMID: 23237794
-
Synthesis of a stable analog of the phosphorylated form of CheY: phosphono-CheY.Methods Enzymol. 2007;422:338-51. doi: 10.1016/S0076-6879(06)22016-0. Methods Enzymol. 2007. PMID: 17628147
-
Synthesis and biochemical characterization of an analogue of CheY-phosphate, a signal transduction protein in bacterial chemotaxis.Biochemistry. 1998 Sep 29;37(39):13674-80. doi: 10.1021/bi9806293. Biochemistry. 1998. PMID: 9753454
Cited by
-
Phosphonopeptides containing free phosphonic groups: recent advances.RSC Adv. 2020 Jul 9;10(43):25898-25910. doi: 10.1039/d0ra04655h. eCollection 2020 Jul 3. RSC Adv. 2020. PMID: 35518575 Free PMC article. Review.
References
-
- Lam KH, Lam WW, Wong JY, Chan LC, Kotaka M, Ling TK, Jin DY, Ottemann KM, Au SW (2013) Structural basis of FliG–FliM interaction in Helicobacter pylori . Mol Microbiol 88:798–812. - PubMed
-
- Bischoff DS, Bourret RB, Kirsch ML, Ordal GW (1993) Purification and characterization of Bacillus subtilis CheY. Biochemistry 32:9256–9261. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

