An insider's perspective: Bacteroides as a window into the microbiome
- PMID: 28440278
- PMCID: PMC5679392
- DOI: 10.1038/nmicrobiol.2017.26
An insider's perspective: Bacteroides as a window into the microbiome
Abstract
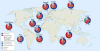
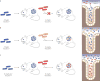
Over the last decade, our appreciation for the contribution of resident gut microorganisms-the gut microbiota-to human health has surged. However, progress is limited by the sheer diversity and complexity of these microbial communities. Compounding the challenge, the majority of our commensal microorganisms are not close relatives of Escherichia coli or other model organisms and have eluded culturing and manipulation in the laboratory. In this Review, we discuss how over a century of study of the readily cultured, genetically tractable human gut Bacteroides has revealed important insights into the biochemistry, genomics and ecology that make a gut bacterium a gut bacterium. While genome and metagenome sequences are being produced at breakneck speed, the Bacteroides provide a significant 'jump-start' on uncovering the guiding principles that govern microbiota-host and inter-bacterial associations in the gut that will probably extend to many other members of this ecosystem.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. - PubMed
-
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. - PubMed
-
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

