Targeting ASIC3 for Relieving Mice Fibromyalgia Pain: Roles of Electroacupuncture, Opioid, and Adenosine
- PMID: 28440280
- PMCID: PMC5404229
- DOI: 10.1038/srep46663
Targeting ASIC3 for Relieving Mice Fibromyalgia Pain: Roles of Electroacupuncture, Opioid, and Adenosine
Abstract
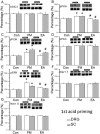
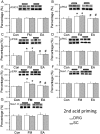
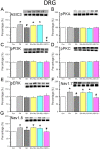
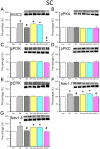
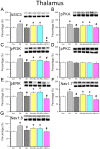
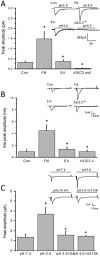
Many scientists are seeking better therapies for treating fibromyalgia (FM) pain. We used a mouse model of FM to determine if ASIC3 and its relevant signaling pathway participated in FM pain. We demonstrated that FM-induced mechanical hyperalgesia was attenuated by electroacupuncture (EA). The decrease in fatigue-induced lower motor function in FM mice was also reversed by EA. These EA-based effects were abolished by the opioid receptor antagonist naloxone and the adenosine A1 receptor antagonist rolofylline. Administration of opioid receptor agonist endomorphin (EM) or adenosine A1 receptor agonist N6-cyclopentyladenosine (CPA) has similar results to EA. Similar results were also observed in ASIC3-/- or ASIC3 antagonist (APETx2) injected mice. Using western blotting, we determined that pPKA, pPI3K, and pERK were increased during a dual acidic injection priming period. Nociceptive receptors, such as ASIC3, Nav1.7, and Nav1.8, were upregulated in the dorsal root ganglion (DRG) and spinal cord (SC) of FM mice. Furthermore, pPKA, pPI3K, and pERK were increased in the central thalamus. These aforementioned mechanisms were completely abolished in ASIC3 knockout mice. Electrophysiological results also indicated that acid potentiated Nav currents through ASIC3 and ERK pathway. Our results highlight the crucial role of ASIC3-mediated mechanisms in the treatment of FM-induced mechanical hyperalgesia.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Vierck C. J. Jr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain 124, 242–263 (2006). - PubMed
-
- Roth T., Arnold L. M., Garcia-Borreguero D., Resnick M. & Clair A. G. A review of the effects of pregabalin on sleep disturbance across multiple clinical conditions. Sleep medicine reviews 18, 261–271 (2014). - PubMed
-
- Smith M. T. & Moore B. J. Pregabalin for the treatment of fibromyalgia. Expert opinion on pharmacotherapy 13, 1527–1533 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

