Live cell imaging of mitochondria following targeted irradiation in situ reveals rapid and highly localized loss of membrane potential
- PMID: 28440317
- PMCID: PMC5404225
- DOI: 10.1038/srep46684
Live cell imaging of mitochondria following targeted irradiation in situ reveals rapid and highly localized loss of membrane potential
Abstract
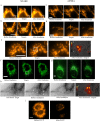
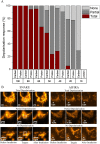
The reliance of all cell types on the mitochondrial function for survival makes mitochondria an interesting target when trying to understand their role in the cellular response to ionizing radiation. By harnessing highly focused carbon ions and protons using microbeams, we have performed in situ live cell imaging of the targeted irradiation of individual mitochondria stained with Tetramethyl rhodamine ethyl ester (TMRE), a cationic fluorophore which accumulates electrophoretically in polarized mitochondria. Targeted irradiation with both carbon ions and protons down to beam spots of <1 μm induced a near instant loss of mitochondrial TMRE fluorescence signal in the targeted area. The loss of TMRE after targeted irradiation represents a radiation induced change in mitochondrial membrane potential. This is the first time such mitochondrial responses have been documented in situ after targeted microbeam irradiation. The methods developed and the results obtained have the ability to shed new light on not just mitochondria's response to radiation but to further elucidate a putative mechanism of radiation induced depolarization and mitochondrial response.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Clarke R. H. & Valentin J. The History of ICRP and the Evolution of its Policies. Annals of the ICRP 39, 75–110, doi: 10.1016/j.icrp.2009.07.009 (2009). - DOI
-
- Watanabe W. et al.. Femtosecond laser disruption of mitochondria in living cells. Medical Laser Application 20, 185–191, doi: 10.1016/j.mla.2005.07.004 (2005). - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

